Abstract
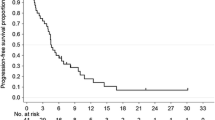
We aimed to study the efficacy and safety of metronomic capecitabine in pretreated elderly patients with advanced gastric cancer. Eligible patients with advanced gastric cancer were treated with capecitabine at a fixed dose 1,000 mg daily (days 1–28 continuously, every 5 weeks) until disease progression or significant toxicity. Tumor response was assessed every 10 weeks by computed tomography scan using Response Evaluation Criteria in solid tumors. In total, 45 patients were enrolled, of whom 43 were evaluated for efficacy and 45 for safety. A median of 3 cycles (range 1–12) were administered. Metronomic chemotherapy had a disease control rate (DCR) at 8 weeks of 51.1% (95% CI 25.7–67.8), and the objective response rate was 20.9% (95% CI 13.1–38.5, 9 of 43 assessable patients). The median time-to-progression and median overall survival were 3.6 months (95% CI: 3.2–4.0 months) and 7.6 months (95% CI 7.0–8.2 months), respectively. Grade II neutropenia and thrombocytopenia were observed in 13.3 and 2.2% of patients, respectively. Grade II/III nonhematological toxicities included diarrhea (4.4%), stomatitis (13.4%), and hand–foot syndrome (15.5%). No grade IV toxicity, neutropenic fever or treatment-related deaths occurred. Metronomic capecitabine was effective and well tolerated as palliative treatment in elderly patients with advanced gastric cancer after fluoropyrimidine-based chemotherapy.


Similar content being viewed by others
References
Chen XM, Chen GY, Wang ZR, Zhu FS, Wang XL, Zhang X. Detection of micrometastasis of gastric carcinoma in peripheral blood circulation. World J Gastroenterol. 2004;10:804–8.
Hu JK, Li CM, Chen XZ, Chen ZX, Zhou ZG, Zhang B, Chen JP. The effectiveness of intravenous 5-fluorouracil-containing chemotherapy after curative resection for gastric carcinoma: a systematic review of published randomized controlled trials. J Chemother. 2007;19:359–75.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev (abstract). 2010;17:3.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA, V325 Study Group. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7.
Lee JL, Kang YK, Kang HJ, Lee KH, Zang DY, Ryoo BY, Kim JG, Park SR, Kang WK, Shin DB, Ryu MH, Chang HM, Kim TW, Baek JH, Min YJ. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer. 2008;19:584–90.
Seol YM, Song MK, Choi YJ, Kim GH, Shin HJ, Song GA, Chung JS, Cho GJ. Oral fluoropyrimidines (capecitabine or S-1) and cisplatin as first line treatment in elderly patients with advanced gastric cancer: a retrospective study. Jpn J Clin Oncol. 2009;39:43–8.
Park SH, Kang WK, Lee HR, Park J, Lee KE, Lee SH, Park JO, Kim K, Kim WS, Chung CW. Docetaxel plus cisplatin as second-line therapy in metastatic or recurrent advanced gastric cancer progressing on 5-fluorouracil-based regimen. Am J Clin Oncol. 2004;27:477–80.
Park SH, Kim YS, Hong J, Park J, Nam E, Cho EK, Shin DB, Lee JH, Lee WK, Chung M. Mitomycin C plus S-1 as second-line therapy in patients with advanced gastric cancer: a noncomparative phase II study. Anticancer Drugs. 2008;19:303–7.
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34:1274–81.
Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, Park JN, Han JY, Kang JH, Lee KS, Cho JY. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15:1344–7.
Koizumi W, Saigenji K, Ujiie S, Terashima M, Sakata Y, Taguchi T, Clinical Study Group of Capecitabine. A pilot phase II study of capecitabine in advanced or recurrent gastric cancer. Oncology. 2003;64:232–6.
Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer, M., Bugat R, Burger U, Garin A, Graeven U, McKendrick J, Maroun J, Marshall J, Osterwalder B, Pérez-Manga G, Rosso R, Rougier P, Schilsky RL. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. 2002;13:566–575.
Emmenegger U, Kerbel RS. Five years of clinical experience with metronomic chemotherapy: achievements and perspectives. Onkologie. 2007;30:606–8.
Petrioli R, Pascucci A, Francini E, Marsili S, Fiaschi AI, Civitelli S, Tanzini G, Battistelli S, Lorenzi M, Roviello F, Francini G. Multidisciplinary oncology group on gastrointestinal tumors. Continuous oral capecitabine at fixed dose in patients older than 75 years with metastatic colorectal and gastric cancer: a study of the Multidisciplinary Oncology Group on Gastrointestinal Tumors. Anticancer Drugs. 2008;19:91–6.
Taguchi T, Nakayama T, Masuda N, Yoshidome K, Akagi K, Nishida Y, Yoshikawa Y, Ogino N, Abe C, Sakamoto J, Noguchi S. Study of low-dose capecitabine monotherapy for metastatic breast cancer. Chemotherapy. 2010;56:166–70.
Steinbild S, Arends J, Medinger M, Häring B, Frost A, Drevs J, Unger C, Strecker R, Hennig J, Mross K. Metronomic antiangiogenic therapy with capecitabine and celecoxib in advanced tumor patients—results of a phase II study. Onkologie. 2007;30:629–35.
Simon R. How large should a phase II trial of a new drug be. Cancer Treat Rep. 1987;71:1079–85.
Wöhrer SS, Raderer M, Hejnal M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol. 2004;15:1585–95.
Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US foot and drug administration. J Clin Oncol. 2004;22:4626–31.
Franchi F, Grassi P, Ferro D, Pigliucci G, De Chicchis M, Castigliani G, Pastore C, Seminara P. Antiangiogenic metronomic chemotherapy and hyperthermia in the palliation of advanced cancer. Eur J Cancer Care (Engl). 2003;16:258–62.
Salem DA, Gado NM, Abdelaziz NN, Essa AE, Abdelhafeez ZM, Kamel TH. Phase II trial of metronomic chemotherapy as salvage therapy for patients with metastatic breast cancer. J Egypt Natl Canc Inst. 2008;20:134–40.
Kesari S, Schiff D, Doherty L, Gigas DC, Batchelor TT, Muzikansky A, O’Neill A, Drappatz J, Chen-Plotkin AS, Ramakrishna N, Weiss SE, Levy B, Bradshaw J, Kracher J, Laforme A, Black PM, Folkman J, Kieran M, Wen PY. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. 2007;9354–9363.
Penel N, Clisant S, Dansin E, Desauw C, Dégardin M, Mortier L, Vanhuyse M, Bonodeau F, Fournier C, Cazin JL, Adenis A. Megestrol acetate versus metronomic cyclophosphamide in patients having exhausted all effective therapies under standard care. Br J Cancer. 2010;102:1207–12.
Nannini M, Nobili E, Di Cicilia R, Brandi G, Maleddu A, Pantaleo MA, Biasco G. To widen the setting of cancer patients who could benefit from metronomic capecitabine. Cancer Chemother Pharmacol. 2009;64:189–93.
Van Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P, Graeven U, Lokich J, Madajewicz S, Maroun JA, Marshall JL, Mitchell EP, Perez-Manga G, Rougier P, Schmiegel W, Schoelmerich J, Sobrero A, Schilsky RL. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. 2004;90:1190–7.
Comella P, Franco L, Casaretti R, de Portu S, Menditto E. Emerging role of capecitabine in gastric cancer. Pharmacotherapy. 2009;29:318–30.
Calvani N, Orlando L, Nacci A, Sponziello F, Cinefra M, Cinieri S. Metronomic chemotherapy against cancer: from paradigm to clinical practice? Tumori. 2009;95:843–5.
Lokich J. Capecitabine: fixed daily dose and continuous (noncyclic) dosing schedule. Cancer Invest. 2004;22:713–7.
Sun JF, Wu RR, Norris C, Noone AM, Amankwa-Sakyi M, Slack R, Marshall JL. Safety of chronic low-dose capecitabine as maintenance therapy in gastrointestinal cancers. Gastrointest Cancer Res. 2009;3:134–40.
Lichtman SM, Wildiers H, Chatelut E, Steer C, Budman D, Morrison VA, Tranchand B, Shapira I, Aapro M. International society of geriatric oncology chemotherapy taskforce: evaluation of chemotherapy in older patients–an analysis of the medical literature. J Clin Oncol. 2007;25:1832–43.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin vs S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9.
Trumper M, Ross PJ, Cunningham D, Norman AR, Hawkins R, Seymour M, Harper P, Iveson T, Nicolson M, Hickish T. Efficacy and tolerability of chemotherapy in elderly patients with advanced oesophago-gastric cancer: a pooled analysis of three clinical trials. Eur J Cancer. 2006;42:827–34.
Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–22.
Ooyama A, Oka T, Zhao HY, Yamamoto M, Akiyama S, Fukushima M. Anti-angiogenic effect of 5-Fluorouracil-based drugs against human colon cancer xenografts. Cancer Lett. 2008;267:26–36.
Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–65.
Rozados VR, Mainetti LE, Rico MJ, Zacarías Fluck MF, Matar P, Scharovsky OG. The immune response and the therapeutic effect of metronomic chemotherapy with cyclophosphamide. Oncol Res. 2010;18:601–5.
Bocci G, Falcone A, Fioravanti A, Orlandi P, Di Paolo A, Fanelli G, Viacava P, Naccarato AG, Kerbel RS, Danesi R, Del Tacca M, Allegrini G. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br J Cancer. 2008;98:1619–29.
Jurado JM, Sánchez A, Pajares B, Pérez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–6.
Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, Ding YH, Hsu C, Cheng AL. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010;53:126–31.
Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, Pietri E, Colleoni M. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–905.
Acknowledgments
This project was supported by the Shanghai Nature Science Fund, Shanghai, China (0552nm007).
Conflict of interest
The authors declared no conflicts of interest with respect to authorship and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, S., Shen, J., Hong, L. et al. Capecitabine “metronomic” chemotherapy for palliative treatment of elderly patients with advanced gastric cancer after fluoropyrimidine-based chemotherapy. Med Oncol 29, 100–106 (2012). https://doi.org/10.1007/s12032-010-9791-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9791-x