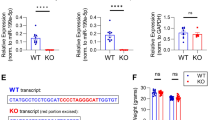
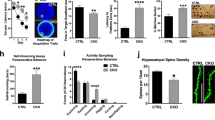
Abstract
Dendritic spines are small, dynamic protrusions along the dendrite that comprise more than 90% of excitatory connections in the brain, making them essential sites for neuronal communication. These synaptic sites change throughout the process of development, reducing in density and shifting morphology as synapses are refined. One important class of dendritic spine regulators is microRNA (miRNA), small-noncoding RNAs that post-transcriptionally regulate gene expression. Several studies suggest that miRNA-324-5p regulates dendritic spine formation. In addition, we have previously shown that miR-324-5p plays a role in seizure and long-term potentiation, both of which involve dendritic spine changes. In this study, we aimed to characterize the role of miRNA-324-5p in developmental spine regulation by assessing the effect of Mir324 knockout (KO) on dendritic spine density and expression of a subset of dendritic proteins at select developmental time points. We show that miR-324-5p expression is developmentally regulated and peaks at 4 weeks of age. We demonstrate that loss of miR-324-5p expression leads to differential changes in both target protein expression and spine density at different time points during development, disrupting the pattern of spine density changes and leading to a premature loss of dendritic spines in KO mice, which is compensated later. Our findings indicate that miR-324-5p plays a role in synaptic refinement across development. Additionally, our data illustrate the importance of context in the study of miRNA, as regulation by and/or of miRNA can vary dramatically across development and in disease.







Similar content being viewed by others
Data Availability
Original data are available upon reasonable request.
Change history
03 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12031-023-02171-6
References
Allen NJ (2014) Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol 30(1):439–463. https://doi.org/10.1146/annurev-cellbio-100913-013053
Berry KP, Nedivi E (2017) Spine dynamics: are they all the same? Neuron 96(1):43–55. https://doi.org/10.1016/j.neuron.2017.08.008
Biever A, Donlin-Asp PG, Schuman EM (2019) Local translation in neuronal processes. Curr Opin Neurobiol 57:141–148. https://doi.org/10.1016/j.conb.2019.02.008
Bredy TW, Lin Q, Wei W, Baker-Andresen D, Mattick JS (2011) MicroRNA regulation of neural plasticity and memory. Neurobiol Learn Mem 96(1):89–94. https://doi.org/10.1016/j.nlm.2011.04.004
Brennan GP, Henshall DC (2020) MicroRNAs as regulators of brain function and targets for treatment of epilepsy. Nat Rev Neurol 16(9):506–519. https://doi.org/10.1038/s41582-020-0369-8
Bulovaite E, Qiu Z, Kratschke M, Zgraj A, Fricker DG, Tuck EJ, Gokhale R, Koniaris B, Jami SA, Merino-Serrais P, Husi E, Mendive-Tapia L, Vendrell M, O’Dell TJ, DeFelipe J, Komiyama NH, Holtmaat A, Fransén E, Grant SGN (2022) A brain atlas of synapse protein lifetime across the mouse lifespan. Neuron 110(24):4057-4073.e8. https://doi.org/10.1016/j.neuron.2022.09.009
Calabrese B, Wilson MS, Halpain S (2006) Development and regulation of dendritic spine synapses. Physiol 21(1):38–47. https://doi.org/10.1152/physiol.00042.2005
Corbin R, Olsson-Carter K, Slack F (2009) The role of microRNAs in synaptic development and function. BMB Rep 42(3):131–135
DeGiosio RA, Grubisha MJ, MacDonald ML, McKinney BC, Camacho CJ, Sweet RA (2022) More than a marker: potential pathogenic functions of MAP2. Front Mol Neurosci 15:974890. https://doi.org/10.3389/fnmol.2022.974890
Di Stefano G, Casoli T, Fattoretti P, Gracciotti N, Solazzi M, Bertoni-Freddari C (2001) Distribution of MAP2 in hippocampus and cerebellum of young and old rats by quantitative immunohistochemistry. J Histochem Cytochem 49(8):1065–1066. https://doi.org/10.1177/002215540104900818
Dogini DB, Avansini S, Schwambach Vieira A, Lopes-Cendes I (2013) MicroRNA regulation and dysregulation in epilepsy. Front Mol Neurosci 7. https://doi.org/10.3389/fncel.2013.00172
Edbauer D, Neilson JR, Foster KA, Wang C-F, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M (2010) Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65(3):373–384. https://doi.org/10.1016/j.neuron.2010.01.005
Feng J, Lu W, Wang D, Ma K, Song Z, Chen N, Sun Y, Du K, Shen M, Cui S, Wang J-H (2017) Barrel cortical neuron integrates triple associated signals for their memory through receiving epigenetic-mediated new synapse innervations. Cereb Cortex 27(12):5858–5871. https://doi.org/10.1093/cercor/bhx292
Giusti SA, Vogl AM, Brockmann MM, Vercelli CA, Rein ML, Trümbach D, Wurst W, Cazalla D, Stein V, Deussing JM, Refojo D (2014) MicroRNA-9 controls dendritic development by targeting REST. ELife 3. https://doi.org/10.7554/eLife.02755
Gross C, Yao X, Engel T, Tiwari D, Xing L, Rowley S, Danielson SW, Thomas KT, Jimenez-Mateos EM, Schroeder LM, Pun RYK, Danzer SC, Henshall DC, Bassell GJ (2016) MicroRNA-mediated downregulation of the potassium channel Kv4.2 contributes to seizure onset. Cell Rep 17(1):37–45. https://doi.org/10.1016/j.celrep.2016.08.074
Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ (2011) Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci 31(15):5693–5698. https://doi.org/10.1523/JNEUROSCI.6661-10.2011
Grossman AW, Aldridge GM, Lee KJ, Zeman MK, Jun CS, Azam HS, Arii T, Imoto K, Greenough WT, Rhyu IJ (2010) Developmental characteristics of dendritic spines in the dentate gyrus of Fmr1 knockout mice. Brain Res 1355:221–227. https://doi.org/10.1016/j.brainres.2010.07.090
Hale CR, Sawicka K, Mora K, Fak JJ, Kang JJ, Cutrim P, Cialowicz K, Carroll TS, Darnell RB (2021) FMRP regulates mRNAs encoding distinct functions in the cell body and dendrites of CA1 pyramidal neurons. ELife 10, e71892. https://doi.org/10.7554/eLife.71892
Hayman DJ, Modebadze T, Charlton S, Cheung K, Soul J, Lin H, Hao Y, Miles CG, Tsompani D, Jackson RM, Briggs MD, Piróg KA, Clark IM, Barter MJ, Clowry GJ, LeBeau FEN, Young DA (2021) Increased hippocampal excitability in miR-324-null mice. Sci Rep 11(1):10452. https://doi.org/10.1038/s41598-021-89874-1
Hu Z, Li Z (2017) MiRNAs in synapse development and synaptic plasticity. Curr Opin Neurobiol 45:24–31. https://doi.org/10.1016/j.conb.2017.02.014
Hurtado AP, Rengifo AC, Torres-Fernández O (2015) Immunohistochemical overexpression of MAP-2 in the cerebral cortex of rabies-infected mice. Int J Morphol 33(2):465–470. https://doi.org/10.4067/S0717-95022015000200010
Kaufmann WE, MacDonald SM, Altamura CR (2000) Dendritic cytoskeletal protein expression in mental retardation: an immunohistochemical study of the neocortex in Rett syndrome. Cereb Cortex 10(10):992–1004. https://doi.org/10.1093/cercor/10.10.992
Kim J, Jung S-C, Clemens AM, Petralia RS, Hoffman DA (2007) Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 54(6):933–947. https://doi.org/10.1016/j.neuron.2007.05.026
Kim Y, Jang Y-N, Kim J-Y, Kim N, Noh S, Kim H, Queenan BN, Bellmore R, Mun JY, Park H, Rah JC, Pak DTS, Lee KJ (2020) Microtubule-associated protein 2 mediates induction of long-term potentiation in hippocampal neurons. FASEB J 34(5):6965–6983. https://doi.org/10.1096/fj.201902122RR
Koester SK, Dougherty JD (2022) A proposed role for interactions between argonautes, miRISC, and RNA binding proteins in the regulation of local translation in neurons and glia. J Neurosci 42(16):3291–3301. https://doi.org/10.1523/JNEUROSCI.2391-21.2022
Lee HY, Ge W-P, Huang W, He Y, Wang GX, Rowson-Baldwin A, Smith SJ, Jan YN, Jan LY (2011) Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron 72(4):630–642. https://doi.org/10.1016/j.neuron.2011.09.033
Lei Z, Wang D, Chen N, Ma K, Lu W, Song Z, Cui S, Wang J-H (2017) Synapse innervation and associative memory cell are recruited for integrative storage of whisker and odor signals in the barrel cortex through miRNA-mediated processes. Front Cell Neurosci 11. https://doi.org/10.3389/fncel.2017.00316
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46(6):957–967. https://doi.org/10.1016/j.immuni.2017.06.006
Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C, Rheinheimer S, Meder B, Stähler C, Meese E, Keller A (2016) Distribution of miRNA expression across human tissues. Nucleic Acids Res 44(8):3865–3877. https://doi.org/10.1093/nar/gkw116
Manders EMM, Verbeek FJ, Aten JA (1993) Measurement of co-localization of objects in dual-colour confocal images. J Microsc 169(3):375–382. https://doi.org/10.1111/j.1365-2818.1993.tb03313.x
Moreau MP, Bruse SE, Jornsten R, Liu Y, Brzustowicz LM (2013) Chronological changes in MicroRNA expression in the developing human brain. PloS one 8(4):e60480. https://doi.org/10.1371/journal.pone.0060480
Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, Warren ST, Bassell GJ (2011) Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation and mGluR signaling. Mol Cell 42(5):673–688. https://doi.org/10.1016/j.molcel.2011.05.006
Nestor MW, Hoffman DA (2012) Differential cycling rates of Kv4.2 channels in proximal and distal dendrites of hippocampal CA1 pyramidal neurons. Hippocampus 22(5):969–980. https://doi.org/10.1002/hipo.20899
Ota Y, Zanetti AT, Hallock RM (2013) The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast 2013. https://doi.org/10.1155/2013/185463
Parkins EV, Brager DH, Rymer JK, Burwinkel JM, Rojas D, Tiwari D, Hu Y-C, Gross C (2023) MiR-324–5p regulates the structure of dendritic spines and is essential for hippocampal long-term potentiation (p. 2023.07.19.549725). bioRxiv. https://doi.org/10.1101/2023.07.19.549725
Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS (2004) KChIPs and Kv4 α subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci 24(36):7903–7915. https://doi.org/10.1523/JNEUROSCI.0776-04.2004
Riccomagno MM, Kolodkin AL (2015) Sculpting neural circuits by axon and dendrite pruning. Annu Rev Cell Dev Biol 31:779–805. https://doi.org/10.1146/annurev-cellbio-100913-013038
Roy B, Jacobson A (2013) The intimate relationships of mRNA decay and translation. Trends Genet 29(12):691–699. https://doi.org/10.1016/j.tig.2013.09.002
Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439(7074). https://doi.org/10.1038/nature04367
Smalheiser NR, Lugli G (2009) MicroRNA regulation of synaptic plasticity. NeuroMol Med 11(3):133–140. https://doi.org/10.1007/s12017-009-8065-2
Sorra KE, Harris KM (2000) Overview on the structure, composition, function, development, and plasticity of hippocampal dendritic spines. Hippocampus 10(5):501–511. https://doi.org/10.1002/1098-1063(2000)10:5%3C501::AID-HIPO1%3E3.0.CO;2-T
Sun C, Zhu L, Ma R, Ren J, Wang J, Gao S, Yang D, Ning K, Ling B, Lu B, Chen X, Xu J (2019) Astrocytic miR-324-5p is essential for synaptic formation by suppressing the secretion of CCL5 from astrocytes. Cell Death Dis 10(2):141. https://doi.org/10.1038/s41419-019-1329-3
Thomas KT, Gross C, Bassell GJ (2018) MicroRNAs sculpt neuronal communication in a tight balance that is lost in neurological disease. Front Cell Neurosci 11. https://doi.org/10.3389/fnmol.2018.00455
Tiwari D, Brager DH, Rymer JK, Bunk AT, White AR, Elsayed NA, Krzeski JC, Snider A, Schroeder Carter LM, Danzer SC, Gross C (2019) MicroRNA inhibition upregulates hippocampal A-type potassium current and reduces seizure frequency in a mouse model of epilepsy. Neurobiol Dis 130:104508. https://doi.org/10.1016/j.nbd.2019.104508
West AE (2019) Activity-dependent transcription collaborates with local dendritic translation to encode stimulus-specificity in the genome binding of NPAS4. Neuron 104(4):634–636. https://doi.org/10.1016/j.neuron.2019.10.022
White AR, Tiwari D, MacLeod MC, Danzer SC, Gross C (2020) PI3K isoform-selective inhibition in neuron-specific PTEN-deficient mice rescues molecular defects and reduces epilepsy-associated phenotypes. Neurobiol Dis 144:105026. https://doi.org/10.1016/j.nbd.2020.105026
Won S, Levy JM, Nicoll RA, Roche KW (2017) MAGUKs: multifaceted synaptic organizers. Curr Opin Neurobiol 43:94–101. https://doi.org/10.1016/j.conb.2017.01.006
Wu R, Cui S, Wang J-H (2020) MiRNA-324/-133a essential for recruiting new synapse innervations and associative memory cells in coactivated sensory cortices. Neurobiol Learn Mem 172:107246. https://doi.org/10.1016/j.nlm.2020.107246
Yan F, Gao Z, Chen P, Huang L, Wang D, Chen N, Wu R, Feng J, Cui S, Lu W, Wang J-H (2016) Coordinated plasticity between barrel cortical glutamatergic and GABAergic neurons during associative memory [Research Article]. Neural Plasticity; Hindawi Volume 2016, Article ID 5648390. https://doi.org/10.1155/2016/5648390
Acknowledgements
The authors would like to thank Nada Elsayed and Lindsay M. Schroeder for technical assistance, the entire Gross Lab for thoughtful comments and discussions, as well as the CCHMC Confocal Imaging Core (RRID: SCR_022628) and the CCHMC Veterinary Services team for their dedicated support.
Funding
This research was funded by National Institutes of Health grants R01NS092705 (to CG), R01NS107453 (to CG), R21NS126740 (to CG, DT), T32NS007453 (to EVP), a CCHMC Research Innovation/Pilot Funding Award (to CG), and the Cincinnati Children’s Research Foundation (to all authors).
Author information
Authors and Affiliations
Contributions
Emma Parkins, Durgesh Tiwari, and Christina Gross contributed to the study conception. Emma Parkins, Yueh-Chiang Hu, and Christina Gross contributed to the study design. Emma Parkins, John Burwinkel, Ruvi Ranatunga, and Sarah Yaser performed and analyzed experiments. Emma Parkins wrote the first full draft of the manuscript, which was edited by Christina Gross and read and approved by all authors.
Corresponding author
Ethics declarations
Competing Interests
C.G. is co-Inventor on US patent 9,932,585 B2. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In the originally published version of this article, the labels in panel b of Figure 3 were shifted and became illegible. Figure 3 has been edited to ensure correct display of labels.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parkins, E.V., Burwinkel, J.M., Ranatunga, R. et al. Age-Dependent Regulation of Dendritic Spine Density and Protein Expression in Mir324 KO Mice. J Mol Neurosci 73, 818–830 (2023). https://doi.org/10.1007/s12031-023-02157-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-023-02157-4