Abstract
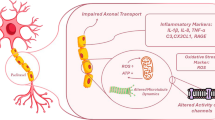
KLS-13019, a novel devised cannabinoid-like compound, was explored for anti-inflammatory actions in dorsal root ganglion cultures relevant to chemotherapy-induced peripheral neuropathy (CIPN). Time course studies with 3 µM paclitaxel indicated > 1.9-fold increases in immunoreactive (IR) area for cell body GPR55 after 30 min as determined by high content imaging. To test for reversibility of paclitaxel-induced increases in GPR55, cultures were treated for 8 h with paclitaxel alone and then a dose response to KLS-13019 added for another 16 h. This “reversal” paradigm indicated established increases in cell body GPR55 IR areas were decreased back to control levels. Because GPR55 had previously reported inflammatory actions, IL-1β and NLRP3 (inflammasome-3 marker) were also measured in the “reversal” paradigm. Significant increases in all inflammatory markers were produced after 8 h of paclitaxel treatment alone that were reversed to control levels with KLS-13019 treatment. Accompanying studies using alamar blue indicated that decreased cellular viability produced by paclitaxel treatment was reverted back to control levels by KLS-13019. Similar studies conducted with lysophosphatidylinositol (GPR55 agonist) in DRG or hippocampal cultures demonstrated significant increases in neuritic GPR55, NLRP3 and IL-1β areas that were reversed to control levels with KLS-13019 treatment. Studies with a human GPR55-β-arrestin assay in Discover X cells indicated that KLS-13019 was an antagonist without agonist activity. These studies indicated that KLS-13019 has anti-inflammatory properties mediated through GPR55 antagonist actions. Together with previous studies, KLS-13019 is a potent neuroprotective, anti-inflammatory cannabinoid with therapeutic potential for high efficacy treatment of neuropathic pain.








Similar content being viewed by others
Data Availability
Data analyzed in these studies are available from the corresponding author upon reasonable request.
References
Bih CI, Chen T, Nunn AVW, Bazelot M, Dallas M, Whalley BJ (2015) Molecular targets of cannabidiol in neurological disorders. Neurotherap 12:699–730
Bonezzi C, Demartini L (1999) Treatment options in postherpetic neuralgia. Acta Neurol Scand 100(s173):25–35
Boyette-Davis JA, Walters ET, Dougherty PM (2015) Mechanisms involved in the development of chemotherapy-induced neuropathy. Pain Manag 5:285–296. https://doi.org/10.2217/pmt.15.19
Brenneman DE, Petkanas D, Kinney WA (2018) Pharmacological comparisons between cannabidiol and KLS-13019. J Mol Neurosci 66:121–134
Brenneman DE, Kinney WA, Ward SJ (2019) Knockdown siRNA targeting the mitochondrial sodium-channel WA exchanger-1 inhibits the protective effects of two cannabinoids against acute paclitaxel toxicity. J Mol Neurosci 68:603–619
Canta A, Pozzi E, Carozzi VA (2015) Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (CIPN). Toxics 3:198–223. https://doi.org/10.3390/toxics3020198
Celsi F, Pizzo P, Brini M, Leo S, Fotino C, Pinton P, Rissuto R (2009) Mitochondria, calcium and cell death: a deadly triad in neurodegeneration. Bioch Biophys Acta 1787:335–344
Chiurchiu GV, Lanuti M, De Bardi M, Battistini L, Maccarrone M (2015) The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int Immunol 27:153–160
Duggett NA, Griffiths LA, McKenna OE, de Santis V et al (2016) Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neurosci 333:13–26
Foss JD, Farkas DJ, Huynh LM, Kinney WA, Brenneman DE, Ward SJ (2021) Behavioral and pharmacological effects of cannabidiol (CBD) and the CBD analogue KLS-13019 in mouse models of pain and reinforcement. Brit J Pharmacol 178:3067–3078
Fumagalli G, Monza L, Cavaletti G, Rigolio R, Meregalli C (2021) Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Frontiers in Immunol 11:1
Gangadharan V, Selvaraj D, Kurejova M, Njoo C, Gritsch S, Skoricova D et al (2013) A novel biological role for the phospholipid lysophosphatidylinositol in nociceptive sensitization via activation of diverse G-protein signaling pathways in sensory neurons in vivo. Pain 154:2801–2812
Guerrero-Alba R, Barragan-Iglesias P, Gonzalez-Hernandez A, Valdez-Morales E, Granado-Soto C-L, m, Rodriguez M, Marichal-Cancino B (2019) Some prospective alternative for treating pain: the endocannabinoid system and its putative receptors GPR18 and GPR55. Frontiers in Pharmacol 9:1–20
Gutierrez-Gutierrez GM, Sereno et al (2010) Chemotherapy-induced peripheral neuropathy: clinical features, diagnosis, prevention and treatment strategies. Clin Transl Oncol 12(2):81–91
Han Y, Smith MT (2013) Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Frontiers in Pharmacol 4:156
Iuchi K, Nishimaki K, Kamimura N, Ohta S (2019) Molecular hydrogen suppresses free-radical-induced cell death by mitigating fatty acid peroxidation and mitochondrial dysfunction. Can J Physiol Pharmacol 10:999–1005
Jiang H, He H, Chen Y, Huang W, Cheng J et al (2017) Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med 214:3219–3238
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Intl J Mol Sci 20:3328
Kinney WA, McDonnell ME, Zhong HM, Liu C, Yang L, Ling W, Qian T, Chen Y, Cai Z, Petkanas D, Brenneman DE (2016) Discovery of KLS-13019, a cannabidiol-derived neuroprotective agent, with improved potency, safety, and permeability. ACS Med Chem Lett 7:424–428
Kodama D, Ono H, Tanabe M (2007) Altered hippocampal long-term potentiation after peripheral nerve injury in mice. Eur J Pharmacol 574:127–132
Krames ES (2014) The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med 15:1669–1685
Kurano M, Kobayashi T, Sakai E, Tsukamoto K, Yatomi Y (2021) Lysophosphatidylinositol, especially albumin-bound form, induces inflammatory cytokines in macrophages. FASEB J 35(6):e21672. https://doi.org/10.1096/fj.202100245R
LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordon MA (2013) Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: implication for chemotherapy-induced peripheral neuropathy. Neurotox 37:231–239
Li X, Wang L, Fang P, Sun Y, Jiang X, Wang H, Yang X-F (2018) Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation. J Biol Chem 293:11033–11045
Makker PGS, Duffy SS, Lees JG, Perera CJ, Tonkin RS et al (2017) Characterization of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS ONE 12(1):e0170814. https://doi.org/10.1371/journal.pone.0170814
Milost J, Bryk M, Starowicz K (2020) Cannabidiol for pain treatment: focus on pharmacology and mechanism of action. Intl J Mol Sci 21:8870
Nezhady MAM, Rivera JC, Chemtob S (2020) Location bias as emerging paradigm in GPCR biology and drug discovery. iScience 23:101643
Okine BN, Mc Laughlin G, Gaspar JC, Harhen B, Roche M, Finn DP (2020) Antinociceptive effects on the GPR55 antagonists CID16020046 injected into the rat anterior cingulate cortex. Neurosci 443:19–29
Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, Wong GY, Mantyh PW (2007) Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 203(1):42–54. https://doi.org/10.1016/j.brainres.2007.06.066. Epub 2007 Jul 17
Rajagopal S, Shenoy SK (2018) GPCR desensitization: acute and prolonged phases. Cell Signal 41:9–16
Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B (2009) Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci 29:2053–2063
Schuelert N, McDougall J (2011) The abnormal cannabidiol analogue O-1602 reduces nociception in a rat model of acute arthritis via the putative cannabinoid receptor GPR55. Neurosci Lett 500:72–76
Smith JA, Slusher BS, Wozniak KM, Farah MH, Smiyun G, Wilson L, Feinstein S, Jordan MA (2016) Structural basis of induction of peripheral neuropathy by microtubule-targeting cancer drugs. Cancer Res 76:5115–5123
Son S, Shim DW, Hwang I, Yu JW (2019) Chemotherapeutic agent paclitaxel mediates priming of NLRP3 inflammasome activation. Front Immunol 10:1108
Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S (2020) Pathogenesis of paclitaxel-induced peripheral neuropathy: a current view of in vitro and in vivo findings using rodent and human model systems. Exp Neurol 324:113121
Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EN, Hughes JP, Chong E, Mander PK et al (2008) The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain 139:225–236
Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J et al (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. PNAS (USA) 114:E7997–E8006
Tyrtyshnaia A, Manzhulo I (2020) Neuropathic pain causes memory deficits and dendrite tree morphology changes in mouse hippocampus. J Pain Res 13:345–354
Ward SJ, Ramirez MD, Neelakantan H, Walker EA (2011) Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth Analg 113:947–950
Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA (2014) Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy. Brit J Pharmacol 171:636–645
Ward SJ, Riggs D, Tuma R, Kinney WA, Petkanas D, Brenneman DE (2017) Neuroprotective and anti-inflammatory effects of KLS-13019 and cannabidiol in in vitro and in vivo models of chemotherapy-induced neuropathic pain. International Cannabinoid Research Society Symposium, June 22, 2017. Montreal, Canada 27:P1-40
Xu Z, Lv XA, Dai Q, Ge YQ, Xu J (2016) Acute upregulation of neuronal mitochondrial type-1 cannabinoid receptor and its role in metabolic defects and neuronal apoptosis after TBI. Mol Brain 9:25
Yamashita A, Oka S, Tanikawa T, Hayashi Y, Nemoto-Sasaki Y, Suigiura T (2013) The actions and metabolism of lysophosphatidylinositol, an endogenous agonist for GPR55. Prostaglandins Other Lipid Mediat 107:103–116
Zeng QZ, Yang F, Li CG, Xu LH, He XH et al (2019) Paclitaxel enhances the innate immunity by promoting NLRP3 inflammasome activation in macrophages. Front Immunol 10:72
Funding
These studies were supported by Grants from the National Institute on Drug Abuse (R41DA044898); (5P30DA013429-20); (RO1DA045698); and the National Institute of Neurological Disorders and Stroke (R42NS120548) of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Douglas Brenneman designed and conducted all experiments using primary neuronal cultures and wrote the draft of the manuscript. William Kinney and Mark McDonnell chemically designed and provided the purified, structurally verified KLS-13019. Pingei Zhao conducted the β-arrestin assays and authored Fig. 7. Mary Abood provided intellectual input on GPR55 and second messenger systems relevant to study design. Sara Jane Ward provided intellectual input on study design pertaining to inflammation. Editing contributions were made by Brenneman, McDonnell, and Ward. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
Drs Brenneman, Kinney and McDonnell are inventors of KLS-13019 and hold international patents on this technology.
Ethics Approval and Consent to Participants
No animal or human subject data were in this work. Animal tissues for primary cultures were purchased from a commercial source.
Informed Consent
No human subjects were in these studies.
Conflict of Interest
Douglas Brenneman, William Kinney, and Mark McDonnell are inventors of KLS-13019 and hold international patents on the technology described.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brenneman, D.E., Kinney, W.A., McDonnell, M.E. et al. Anti-Inflammatory Properties of KLS-13019: a Novel GPR55 Antagonist for Dorsal Root Ganglion and Hippocampal Cultures. J Mol Neurosci 72, 1859–1874 (2022). https://doi.org/10.1007/s12031-022-02038-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-022-02038-2