Abstract
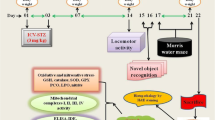
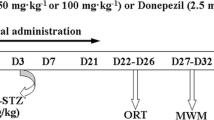
Intracerebroventricular (ICV) microinjection of diabetogenic drug streptozotocin (STZ) in rodents consistently produces a model of sporadic Alzheimer’s disease (sAD) which is characterized by tau pathology and concomitant cognitive decline, insulin resistance, neuroinflammation, oxidative stress, and mitochondrial malfunction. Paeonol is an active phenolic component in some medicinal plants like Cortex Moutan with neuroprotective efficacy via exerting anti-inflammatory and anti-oxidative effects. This study was conducted to assess beneficial effect of paeonol in amelioration of cognitive deficits in ICV STZ rat model of sAD. STZ (3 mg/kg) was microinjected into the lateral ventricles on days 0 and 2, and paeonol was given p.o. at two doses of 25 (low) or 100 (high) mg/kg from day 0 (post-surgery) till day 24 post-STZ. Cognitive performance was evaluated in different tasks, and oxidative stress- and inflammation-related parameters were measured in addition to immunohistochemical assessment of glial fibrillary acidic protein (GFAP) as a marker of astrocytes. Paeonol at the higher dose ameliorated cognitive deficits in Barnes maze, novel object recognition (NOR) task, Y maze, and passive avoidance test. In addition, paeonol partially reversed hippocampal malondialdehyde (MDA), reactive oxygen species (ROS), total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase, glutathione reductase, tumor necrosis factor α (TNFα), interleukin 6 (IL-6), mitochondrial membrane potential (MMP), myeloperoxidase (MPO), and acetylcholinesterase (AChE) activity. Paeonol treatment was also associated with lower hippocampal immunoreactivity for GFAP. This study showed that paeonol can alleviate cognitive disturbances in ICV STZ rat model of sAD via ameliorating neuroinflammation, oxidative stress, mitochondrial dysfunction, and also through its attenuation of astrogliosis.




Similar content being viewed by others
Availability of Data and Materials
Data will be available on editor request.
Abbreviations
- AChE:
-
Acetylcholinesterase
- ADS:
-
Alzheimer’s disease
- GFAP:
-
Glial fibrillary acidic protein
- GSH:
-
Glutathione (reduced form)
- ICV:
-
Intracerebroventricular
- IL-6:
-
Interleukin 6
- MDA:
-
Malondialdehyde
- MMP:
-
Mitochondrial membrane potential
- MPO:
-
Myeloperoxidase
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NOD:
-
Novel object discrimination
- ROS:
-
Reactive oxygen species
- sAD:
-
Sporadic Alzheimer’s disease
- SOD:
-
Superoxide dismutase
- STZ:
-
Streptozotocin
- TAC:
-
Total antioxidant capacity
- TNFα:
-
Tumor necrosis factor α
References
Adki KM, Kulkarni YA (2021) Neuroprotective effect of paeonol in streptozotocin-induced diabetes in rats. Life Sci 271:119–202
Ahshin-Majd S, Zamani S, Kiamari T, Kiasalari Z, Baluchnejadmojarad T, Roghani M (2016) Carnosine ameliorates cognitive deficits in streptozotocin-induced diabetic rats: Possible involved mechanisms. Peptides 86:102–111
Akhtar A, Dhaliwal J, Sah SP (2021) 7,8-Dihydroxyflavone improves cognitive functions in ICV-STZ rat model of sporadic Alzheimer’s disease by reversing oxidative stress, mitochondrial dysfunction, and insulin resistance. Psychopharmacology (Berl)
Alzheimer’s Association (2020) Alzheimer’s disease facts and figures. Alzheimers Dement 16:391–460
Arya A, Sethy NK, Singh SK, Das M, Bhargava K (2013) Cerium oxide nanoparticles protect rodent lungs from hypobaric hypoxia-induced oxidative stress and inflammation. Int J Nanomedicine 8:4507–4520
Baluchnejadmojarad T, Roghani M (2006) Effect of naringenin on intracerebroventricular streptozotocin-induced cognitive deficits in rat: a behavioral analysis. Pharmacology 78(4):193–197
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 239(1):70–76
Bivona G, Lo Sasso B, Gambino CM, Giglio RV, Scazzone C, Agnello L, Ciaccio M (2021) The role of vitamin D as a biomarker in Alzheimer’s disease. Brain Sci 11(3)
Cai Z, Zhao B, Ratka A (2011) Oxidative stress and β-amyloid protein in Alzheimer’s disease. NeuroMol Med 13(4):223–250
Catanesi M, d'Angelo M, Tupone MG, Benedetti E, Giordano A, Castelli V, Cimini A (2020) MicroRNAs dysregulation and mitochondrial dysfunction in neurodegenerative diseases. Int J Mol Sci 21(17)
Chou TC (2003) Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J Pharmacol 139(6):1146–1152
Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL (2017) Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res Ther 9(1):71
Ding J, Yu HL, Ma WW, Xi YD, Zhao X, Yuan LH, Feng JF, Xiao R (2013) Soy isoflavone attenuates brain mitochondrial oxidative stress induced by beta-amyloid peptides 1–42 injection in lateral cerebral ventricle. J Neurosci Res 91(4):562–567
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol Med 11(1):81–128
Fischer R, Maier O (2015) Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev 2015:610813
Folch J, Petrov D, Ettcheto M, Abad S, Sánchez-López E, García ML, Olloquequi J, Beas-Zarate C, Auladell C, Camins A (2016) Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plast 8501693
Guo Y, Du Y, Xie L, Pu Y, Yuan J, Wang Z, Zhang T, Wang B (2020) Effects of Paeonol and Gastroretention tablets of paeonol on experimental gastric ulcers and intestinal flora in rats. Inflammation 43(6):2178–2190
Han F, Xu H, Shen JX, Pan C, Yu ZH, Chen JJ, Zhu XL, Cai YF, Lu YP (2020) RhoA/Rock2/Limk1/cofilin1 pathway is involved in attenuation of neuronal dendritic spine loss by paeonol in the frontal cortex of D-galactose and aluminum-induced Alzheimer’s disease-like rat model. Acta Neurobiol Exp (wars) 80(3):225–244
Jin H, Wang M, Wang J, Cao H, Niu W, Du L (2020) Paeonol attenuates isoflurane anesthesia-induced hippocampal neurotoxicity via modulation of JNK/ERK/P38MAPK pathway and regulates histone acetylation in neonatal rat. J Matern Fetal Neonatal Med 33(1):81–91
Khosravi Z, Sedaghat R, Baluchnejadmojarad T, Roghani M (2019) Diosgenin ameliorates testicular damage in streptozotocin-diabetic rats through attenuation of apoptosis, oxidative stress, and inflammation. Int Immunopharmacol 70:37–46
Kong D, Chen L, Huang W, Zhang Z, Wang L, Zhang F, Zheng S (2020) Combined therapy with ligustrazine and paeonol mitigates hepatic fibrosis through destroying mitochondrial integrity of stellate cell. Am J Transl Res 12(4):1255–1266
Kovacs GG (2017) Cellular reactions of the central nervous system. Handb Clin Neurol 145:13–23
Lin C, Lin HY, Chen JH, Tseng WP, Ko PY, Liu YS, Yeh WL, Lu DY (2015) Effects of paeonol on anti-neuroinflammatory responses in microglial cells. Int J Mol Sci 16(4):8844–8860
Liu J, Feng L, Ma D, Zhang M, Gu J, Wang S, Fu Q, Song Y, Lan Z, Qu R, Ma S (2013) Neuroprotective effect of paeonol on cognition deficits of diabetic encephalopathy in streptozotocin-induced diabetic rat. Neuroscience letters 549:63–68
Ma W, Yuan L, Yu H, Ding B, Xi Y, Feng J, Xiao R (2010) Genistein as a neuroprotective antioxidant attenuates redox imbalance induced by beta-amyloid peptides 25–35 in PC12 cells. Int J Dev Neurosci 28(4):289–295
Maccioni RB, Rojo LE, Fernández JA, Kuljis RO (2009) The role of neuroimmunomodulation in Alzheimer’s disease. Ann NY Acad Sci 1153:240–246
Martins M, Silva R, MM Pinto M, Sousa E (2020) Marine natural products, multitarget therapy and repurposed agents in Alzheimer’s disease. Pharmaceuticals (Basel) 13(9)
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primers 1:15056
McGeer EG, McGeer PL (2003) Inflammatory processes in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 27(5):741–749
Mohamad Nasir NF, Zainuddin A, Shamsuddin S (2018) Emerging roles of Sirtuin 6 in Alzheimer’s disease. J Mol Neurosci 64(2):157–161
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol 33(11):1801–1807
Moura ELR, Dos Santos H, Celes APM, Bassani TB, Souza LC, Vital M (2020) Effects of a nutritional formulation containing caprylic and capric acid, phosphatidylserine, and docosahexaenoic acid in Streptozotocin-lesioned rats. J Alzheimers Dis Rep 4(1):353–363
Nasri S, Roghani M, Baluchnejadmojarad T, Balvardi M, Rabani T (2012) Chronic cyanidin-3-glucoside administration improves short-term spatial recognition memory but not passive avoidance learning and memory in streptozotocin-diabetic rats. Phytother Res 26(8):1205–1210
Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA (2006) Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol 65(7):631–641
Oba H, Kadoya Y, Okamoto H, Matsuoka T, Abe Y, Shibata K, Narumoto J (2021) The economic burden of dementia: evidence from a survey of households of people with dementia and their caregivers. Int J Environ Res Public Health 18(5)
Ojha S, Javed H, Azimullah S, Abul Khair SB, Haque ME (2015) Neuroprotective potential of ferulic acid in the rotenone model of Parkinson’s disease. Drug Des Devel Ther 9:5499–5510
Olszewska-Słonina DM, Mątewski D, Czajkowski R, Olszewski KJ, Woźniak A, Odrowąż-Sypniewska G, Lis K, Musiałkiewicz D, Kowaliszyn B (2011) The concentration of thiobarbituric acid reactive substances (TBARS) and paraoxonase activity in blood of patients with osteoarthrosis after endoprosthesis implantation. Med Sci Monit 17(9):CR498–CR504
Pulli B, Ali M, Forghani R, Schob S, Hsieh KL, Wojtkiewicz G, Linnoila JJ, Chen JW (2013) Measuring myeloperoxidase activity in biological samples. PLoS One 8(7):e67976
Qiu C, Yang LD, Yu W, Tian DD, Gao MR, Wang WJ, Li XB, Wu YM, Wang M (2021) Paeonol ameliorates CFA-induced inflammatory pain by inhibiting HMGB1/TLR4/NF-κB p65 pathway. Metab Brain Dis 36(2):273–283
Ramazi S, Fahanik-Babaei J, Mohamadi-Zarch SM, Tashakori-Miyanroudi M, Nourabadi D, Nazari-Serenjeh M, Roghani M, Baluchnejadmojarad T (2020) Neuroprotective and anticonvulsant effects of sinomenine in kainate rat model of temporal lobe epilepsy: Involvement of oxidative stress, inflammation and pyroptosis. J Chem Neuroanat 108:101800
Rani R, Kumar A, Jaggi AS, Singh N (2021) Pharmacological investigations on efficacy of Phlorizin a sodium-glucose co-transporter (SGLT) inhibitor in mouse model of intracerebroventricular streptozotocin induced dementia of AD type. J Basic Clin Physiol Pharmacol
Salkovic-Petrisic M, Knezovic A, Hoyer S, Riederer P (2013) What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer's disease, about the therapeutic strategies in Alzheimer’s research. J Neural Transm (Vienna, Austria: 1996) 120(1):233–252
Sharma Y, Garabadu D (2020) Ruthenium red, mitochondrial calcium uniporter inhibitor, attenuates cognitive deficits in STZ-ICV challenged experimental animals. Brain Res Bull 164(121–135
Shi X, Chen YH, Liu H, Qu HD (2016) Therapeutic effects of paeonol on methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid-induced Parkinson’s disease in mice. Mol Med Rep 14(3):2397–2404
Simpson DSA, Oliver PL (2020) ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants (Basel, Switzerland) 9(8)
Singh B, Sharma B, Jaggi AS, Singh N (2013) Attenuating effect of lisinopril and telmisartan in intracerebroventricular streptozotocin induced experimental dementia of Alzheimer’s disease type: possible involvement of PPAR-γ agonistic property. J Renin Angiotensin Aldosterone Syst 14(2):124–136
Stuart SA, Robertson JD, Marrion NV, Robinson ES (2013) Chronic pravastatin but not atorvastatin treatment impairs cognitive function in two rodent models of learning and memory. PLoS One 8(9):e75467
Uddin MS, Mamun AA, Hossain MS, Akter F, Iqbal MA, Asaduzzaman M (2016) Exploring the effect of Phyllanthus emblica L. on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: promising natural gift for the mitigation of Alzheimer’s disease. Ann Neurosci 23(4):218–229
Verma V, Singh D, Kh R (2020) Sinapic acid alleviates oxidative stress and neuro-inflammatory changes in sporadic model of Alzheimer’s disease in Rats. Brain Sci 10(12)
Walker JM (1996) The Bicinchoninic Acid (BCA) Assay for protein quantitation. In: Walker JM (ed) The protein protocols handbook. Humana Press, Totowa, NJ, pp 11–14
Wang D, Liu L, Li S, Wang C (2018) Effects of paeoniflorin on neurobehavior, oxidative stress, brain insulin signaling, and synaptic alterations in intracerebroventricular streptozotocin-induced cognitive impairment in mice. Physiol Behav 191:12–20
Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochem Biophys Acta 1842(8)1240–1247
Wang X, Zhu G, Yang S, Wang X, Cheng H, Wang F, Li X, Li Q (2011) Paeonol prevents excitotoxicity in rat pheochromocytoma PC12 cells via downregulation of ERK activation and inhibition of apoptosis. Planta Med 77(15):1695–1701
Wopara I, Modo EU, Adebayo OG, Mobisson SK, Nwigwe JO, Ogbu PI, Nwankwo VU, Ejeawa CU (2021) Anxiogenic and memory impairment effect of food color exposure: upregulation of oxido-neuroinflammatory markers and acetyl-cholinestrase activity in the prefrontal cortex and hippocampus. Heliyon 7(3):e06378–e06378
Yang N, Guan QW, Chen FH, Xia QX, Yin XX, Zhou HH, Mao XY (2020) Antioxidants targeting mitochondrial oxidative stress: promising neuroprotectants for epilepsy. Oxid Med Cell Longev 6687185
Ye M, Yi Y, Wu S, Zhou Y, Zhao D (2017) Role of paeonol in an astrocyte model of Parkinson’s disease. Medical science monitor: international medical journal of experimental and clinical research 23:4740–4748
Zameer S, Kaundal M, Vohora D, Ali J, Kalam Najmi A, Akhtar M (2019) Ameliorative effect of alendronate against intracerebroventricular streptozotocin induced alteration in neurobehavioral, neuroinflammation and biochemical parameters with emphasis on Aβ and BACE-1. Neurotoxicology 70:122–134
Zappa Villar MF, López Hanotte J, Falomir Lockhart E, Trípodi LS, Morel GR, Reggiani PC (2018) Intracerebroventricular streptozotocin induces impaired Barnes maze spatial memory and reduces astrocyte branching in the CA1 and CA3 hippocampal regions. J Neural Transm (vienna) 125(12):1787–1803
Zappa Villar MF, López Hanotte J, Pardo J, Morel GR, Mazzolini G, García MG, Reggiani PC (2020) Mesenchymal stem cells therapy improved the Streptozotocin-induced behavioral and Hippocampal impairment in rats. Mol Neurobiol 57(2):600–615
Zhao B, Shi QJ, Zhang ZZ, Wang SY, Wang X, Wang H (2018) Protective effects of paeonol on subacute/chronic brain injury during cerebral ischemia in rats. Exp Ther Med 15(4):3836–3846
Zhong SZ, Ge QH, Qu R, Li Q, Ma SP (2009) Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by d-galactose in ICR mice. J Neurol Sci 277(1–2):58–64
Zhu Y, Peng L, Hu J, Chen Y, Chen F (2019) Current anti-Alzheimer’s disease effect of natural products and their principal targets. J Integr Neurosci 18(3):327–339
Funding
This research study was the results of PhD student thesis project that was approved by Faculty of Basic Sciences (Shahed University, Tehran, Iran) in 2020.
Author information
Authors and Affiliations
Contributions
Z.K. and M.R. designed the study, supervised conductance of experiments, and prepared the manuscript. M.R. performed statistical analysis of data. A.TB. performed experiments and helped in manuscript writing.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All experimental procedures of this study were conducted under ethical committee supervision of Shahed University (Tehran, Iran) that was in accordance to NIH guidelines for the care and use of laboratory animals. All efforts were made to minimize number of animals and to lower their sufferings. Present study was approved by Institutional Ethics Committee of the Shahed University (Approval ID: IR.SHAHED.REC.1399.042).
Consent for Publication
All authors have read the manuscript and approved its submission.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tayanloo-Beik, A., Kiasalari, Z. & Roghani, M. Paeonol Ameliorates Cognitive Deficits in Streptozotocin Murine Model of Sporadic Alzheimer’s Disease via Attenuation of Oxidative Stress, Inflammation, and Mitochondrial Dysfunction. J Mol Neurosci 72, 336–348 (2022). https://doi.org/10.1007/s12031-021-01936-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01936-1