Abstract
Oligodendrocyte precursor cells (OPCs) proliferation and differentiation are essential for remyelination after white matter injury. Astrocytes could promote oligodendrogenesis after white matter damage whereas the underlying mechanisms are unknown. In this study, the role of astrocytic connexin43 (Cx43) hemichannels involved in OPC proliferation and differentiation in chronic hypoxia was evaluated. In an astrocyte-OPC co-culture chronic hypoxia model, OPCs became proliferative but failed to mature into oligodendrocytes. Application of astrocytic Cx43 blockers attenuated astrocyte activation, suppressed Cx43 hemichannel uptake activity and glutamate release induced by hypoxia, as well as improved OPC differentiation. Moreover, AMPA but not NMDA glutamate receptor antagonist rescued OPC differentiation in hypoxia. In conclusion, these findings suggested that astrocytic Cx43 hemichannel inhibition could potentially improve OPC maturation by attenuating AMPAR-mediated glutamate signaling. Astrocytic Cx43 hemichannels could serve as a potential therapeutic target for remyelination after chronic hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oligodendrocyte precursor cells (OPCs) are immature forms of oligodendrocytes (OLs) and play essential roles in remyelination after white matter injury. Remyelination involves proliferation, migration, and differentiation of OPCs, which is tightly regulated by a complex of intrinsic and extrinsic factors (Rowitch and Kriegstein 2010), including a number of neurotransmitters (De Angelis et al. 2012; Gudz et al. 2006; Wake et al. 2011; Zonouzi et al. 2011) and neurotrophins (Girard et al. 2005; McTigue et al. 1998; Murtie et al. 2005; Woodruff et al. 2004).
Astrocytes have been believed to be important in oligodendrogenesis following white matter damage (Miyamoto et al. 2015). In mammalian, astrocytes couple to neighboring cells via gap junction channels (connexin 43, connexin 30, etc), forming extensive intercellular networks. Particularly, astrocytes connect oligodendrocytes by forming gap junction. Cx43 is predominant in astrocytes, and it can form hemichannels which can release ATP, glutamate, and other small molecules under ischemic conditions (Butt et al. 2014; Li et al. 2015; Rouach et al. 2002; Schulz et al. 2015). Astrocytic Cx43 has been shown required for OPC proliferation and differentiation, which is critical for myelin maintenance (Niu et al. 2016; Orthmann-Murphy et al. 2008; Tress et al. 2012). Deletion of astrocytic Cx43 inhibited OPC proliferation (Niu et al. 2016) while Cx43/Cx32 double-knockout mice developed white matter damage at early age (Tress et al. 2012). Our previous study showed that knockout of astrocytic Cx43 significantly attenuated neuronal loss in MCAO models (Xie et al. 2011). However, it has not been studied if inhibiting astrocytic Cx43 is also protective for myelin maintanence and remyelination after ischemic injury. In the current study, we test if astrocytic Cx43 hemichannels are involved in OPC proliferation and differentiation during chronic hypoxia in astrocyte-OPC co-culture model.
Materials and Methods
Astrocyte and Oligodendroglia Cell Cultures
Primary astrocyte cultures were prepared as previously described (Rovegno et al. 2015; Wu et al. 2013; Yi et al. 2017) with minor modifications. Briefly, P2 newborn C57BL/6 mice cortices were dissected with the scissors. After removing meninges, the cortices were mechanically dissociated into small fragments and digested with 0.25% trypsin (Invitrogen, USA) for 5 min at 37 °C. Then, the cells were centrifuged and suspended in Dulbecco’s Modified Eagle’s Medium and Ham’s F-12 (DMEM/F12, Hyclone, USA). Next, the isolated cells were seeded at a density of 1 × 106 per 25-cm2 flask which was coated with poly-D-lysine (Sigma-Aldrich, USA) and added DMEM/F12 supplemented with 10% fetal bovine serum (FBS, Hyclone, USA). These cultures were kept at 37 °C in an incubator (Thermo Fisher Scientific, USA) containing 95% air and 5% CO2. Purified astrocytes were obtained from primary sub-confluent cultures after removing microglia cells by shaking at 220 rpm and pre-plating for 30 min. Purified astrocytes were seeded at 3 × 104/cm2 onto poly-D-lysine-coated glass coverslips and at 3 × 105 into six-well plates. All media were changed every 2 days until the next experiment was performed. In purified cultures, more than 96% cells were glial fibrillary acidic protein (GFAP)-positive and IBA-1-positive cells were less than 3%.
The OPC cultures were prepared as previously described (Miyamoto et al. 2013; Niu et al. 2012) with minor modifications. Briefly, the mixed glial cells were isolated from P2 newborn C57BL/6 mice cerebrum and cultured with DMEM/high glucose (Hyclone, USA) supplemented with 20% FBS. Half of the culture medium was changed every other day. When the cultures were enriched with OPC cells (at 10–12 days), mixed cells were shaken (220 rpm, 2 h) to remove microglia and then shaken at 250 rpm at 37 °C for 18–20 h to harvest purified OPCs. Up to 97% harvested OPCs were positive platelet-derived growth factor receptor-α (PDGFR-α). Purified OPCs were plated on top of astrocytes at 3 × 104 cells per well to construct an astrocyte-OPC co-culture system. To differentiate OPCs into mature OLs, purified OPCs or co-culture system were incubated with DMEM/F12 containing N2 (1%; Gibco, USA), B27 (1%; Gibco, USA), CNTF (10 ng ml−1; Peprotech, USA), T3 (50 ng ml−1; Sigma-Aldrich, USA), and 5% FBS, for 7 days.
Chronic Hypoxia Model
To mimic mild chronic hypoxic conditions, astrocyte-OPC co-culture cells were incubated with non-lethal CoCl2 (5 μM; Sigma-Aldrich, USA) as described previously (Miyamoto et al. 2013, 2015) during OPC differentiation period. Chronic hypoxic conditions were confirmed by enhanced expression of hypoxia-inducible factor 1-alpha (HIF-1α), which is an identified marker indicating hypoxic injury (Miyamoto et al. 2015).
Immunofluorescence Staining
Cells were washed with 0.01 M phosphate-buffered saline (PBS) 3 times and fixed with 4% paraformaldehyde for 15 min. Nonspecific binding was blocked with 10% bovine serum albumin for 1 h at room temperature (RT). Cells were then incubated overnight at 4 °C with primary antibodies diluted in PBS containing 5% bovine serum albumin. The primary antibodies include anti-glial fibrillary acidic protein (anti-GFAP; 1: 200; 3670, Cell Signaling technology, USA), anti-connexin-43 (anti-Cx43; 1: 200; AB1727, Millipore, USA), anti-myelin basic protein (anti-MBP; 1: 200; MAB386, Millipore, USA), anti-oligodendrocyte transcription factor 2 (anti-Olig2; 1: 200; AB9610, Millipore, USA), anti-HIF-1α (1: 100; ab113642, Abcam, USA), and anti-neuroglial antigen 2 (anti-NG2; 1: 200; AB5320, Millipore, USA). For negative controls, the same source as primary antibodies nonspecific IgG was used instead of the primary antibody, and positive controls were performed and compared as those reported previously. After washing three times, the cells were incubated for 1 h at RT with either cyanine3-conjugated goat anti-rabbit IgG (1: 400; 111-165-144, Jackson-ImmunoResearch, USA), fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1: 400; 115-165-146, Jackson-ImmunoResearch, USA), or cyanine3-conjugated goat anti-rat IgG (1: 400; 112-165-143, Jackson-ImmunoResearch, USA). The nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; 1: 1000; D9542, Sigma, USA). The images were captured using a fluorescent microscope (BX51; Olympus, Japan) and a laser confocal microscope (LSM1200; Olympus, Japan). Cells were counted in visual field by Image J.
Western Blotting
Cells were collected in radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor cocktail (Roche, USA), and nucleoproteins were extracted through nuclear extraction kit (p0028, Beyotime, China). Equal amounts of proteins (20–30 μg) of each group were resolved on a 10–15% SDS-PAGE gel and subsequently transferred to a polyvinylidene difluoride membrane (0.22 μm; Millipore, USA) using a semidry Trans-Blot system (Bio-Rad laboratories, USA). The membranes were blocked for 1 h at RT in Tris-buffered saline containing 0.25% Tween-20 (TBST) and 5% non-fat milk. The membranes were then incubated with primary antibodies overnight at 4 °C. Primary antibodies included anti-GFAP (1: 1000, ab7260, Abcam, USA), anti-Cx43 (1: 500), anti-MBP (1: 500), anti-PDGFR-α (1: 1000; 558774, BD Biosciences, USA), anti-HIF-1α (1: 1000), anti-Histone H3 (1:10000; GB13102-1, servicebio, China), anti-Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1: 1000; sc-66163, Stanta Cruz, USA), and anti-β-Actin (1: 1000; sc7210, Stanta Cruz, USA). After washing four times with TBST, the membranes were incubated with Odyssey secondary antibody-IRDye 800-conjugated anti-rabbit IgG or Alexa Fluor 700-conjugated anti-mouse IgG (1: 10000; 925-32211, 926-68070, LI-COR Bioscience, USA) for 1 h at RT. The immunoreactivity of protein bands was captured using an Odyssey IR imaging system (LI-COR Bioscience, USA). The images were analyzed with Image J to obtain the integrated optical density (OD) of signals. GAPDH, β-Actin, and Histone H3 were used as loading controls.
EtBr Uptake Analysis
To assess Cx43 hemichannel activity, cells were incubated with ethidium bromide (EtBr; Sigma-Aldrich, USA) as described (Giaume et al. 2012; Yi et al. 2017) with minor modifications. In brief, co-cultured cells were incubated with 5 μM EtBr for 10 min at 37 °C under different treatment conditions. The cells were then washed three times with PBS and immediately fixed with 4% paraformaldehyde for 15 min at RT. Immunofluorescence was captured using a confocal laser-scanning microscope at an excitation wavelength of 555 nm. The amount of EtBr uptake was evaluated by calculating fluorescence-positive area per visual field with Image J.
5-Ethynyl-20-Deoxyuridine Incorporation Assay
The 5-ethynyl-20-deoxyuridine (EdU) assay kit (Ribobio, China) was used to examine proliferation of OPCs according to manufacturer’s instructions. After being incubated with the proliferation media for 3 days, co-cultures were treated with differentiation media supplemented with EdU (50 μM) for another 24 h at 37 °C, and then washed three times with PBS and fixed with 4% paraformaldehyde for 15 min at RT. Cells with EdU were labeled as instructions. After the cells were incubated with the primary Olig2 (1: 200) and the secondary antibody (fluorescein isothiocyanate-conjugated goat anti-rabbit IgG), images were captured with a confocal laser-scanning microscope. Subsequent image analysis with Image J included cell counts. The OPC proliferation ratio was calculated as the percentage of the number of EdU and Olig2 double-positive cells out of Olig2-positive cells.
Glutamate Concentration Analysis
Glutamate levels were tested with glutamic acid kit (Nanjing Jiancheng Bioengineering Institute, China) in collected culture media of different time points during OPC differentiation stage. The sample OD values were detected with a BioSpectrometer (Eppendorf, Germany) and used to calculate the final concentration of glutamate (μmol L−1).
Statistical Analysis
All the data are presented as the mean ± standard deviation (SD) from at least three independent experiments. Data were analyzed with GraphPad Prism 7 software (GraphPad Software, USA). Statistical comparisons were made using Student’s t test or ANOVA with Tukey post hoc analysis for multiple comparisons. A p value < 0.05 was considered statistically significant.
Results
Astrocyte-OPC Co-Cultures Under Chronic Hypoxic Conditions In Vitro
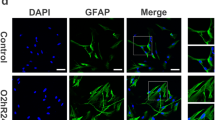
We developed an astrocyte-OPC co-culture model and validated the model by staining specific markers for astrocytes (GFAP, green) and OPCs (NG2, red) (Fig. 1a). In order to mimic chronic hypoxia, co-culture cells were exposed for 7 days to a sublethal dose of CoCl2 (5 μM) in the differentiation media (Miyamoto et al. 2015). Compared to the control, CoCl2 treatment instigated HIF-1α translocation from cytoplasm to nuclei (Fig. 1b) and enhanced HIF-1α expression in nuclei (Fig. 1c, d) in co-cultures, without influencing cells viability (Fig. S1) and inducing cells death (Fig. S2).
Astrocyte and OPC co-culture system of chronic hypoxia model. a Representative images of GFAP (astrocyte marker; green) and NG2 (OPCs marker; red) in the co-culture model. The cell nuclei were stained with DAPI (blue). b Sublethal CoCl2 (5 μM) was administered to mimic prolonged hypoxia in vitro and resulted in the translocation of the hypoxic marker HIF-1α from cytoplasm into to the nuclei in co-cultures. c, d Western blot analysis demonstrated an increased expression of HIF-1α by nucleoprotein analysis, histone H3 was used as a loading control. Scale bar, 50 μm. Data are mean ± SD, **p < 0.01, control group vs CoCl2 group by Student’s t test
Cx43 Inhibitors Attenuated Hypoxia-Induced Astrocyte Activation
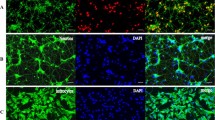
By double immunofluorescent staining, it revealed that Cx43 was co-localized in GFAP-positive astrocytes in co-culture, mainly in cell membrane (Fig. 2a). Compared with normoxia condition, the expression of GFAP and Cx43 was markedly upregulated by 1 day of hypoxia, and gradually but not entirely recovered over the subsequent days (3, 5, and 7 days) (Fig. 2b–d). Gap junction inhibitors meclofenamic acid (MFA, 10 μM) or carbenoxolone (CBX, 50 μM) (Fig. 2a, e–g) could significantly attenuate hypoxia-induced enhancement of GFAP and Cx43 expression at day 2 post-hypoxia treatment, without affecting cells viability (Fig. S1).
Cx43 inhibitors attenuated astrocyte activation under chronic hypoxia. a Representative images of activated astrocytes, with up-regulated GFAP (green) and Cx43 (red), after 2 days of hypoxia as compared to control. MFA (10 μM) and CBX (50 μM) attenuated astrocyte activation. b–d Western blotting confirmed increased GFAP and Cx43 protein levels following hypoxia. e–g MFA and CBX inhibitors decreased hypoxic-induced GFAP and Cx43 protein upregulation in CoCl2-treated cultures. GAPDH was used as a loading control. Scale bar, 50 μm. Data are mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.001, CoCl2 group vs control group by one-way ANOVA; #p < 0.05, ##p < 0.01, MFA or CBX group vs CoCl2 group by one-way ANOVA
Cx43 Inhibitors Rescued the Limited OPC Maturation Under Chronic Hypoxia
The proliferating OPCs were labeled by double-staining EdU and the oligodendroglia lineage marker Olig2. A significant increase in the percentage of EdU+Olig2+ out of Olig2+ cells was observed after hypoxia as compared to normoxic control (Fig. 3a, d), which was inhibited by MFA (10 μM) or CBX (50 μM) treatment (Fig. 3a, d). Meanwhile, the enhanced OPC proliferation was accompanied by failure of the maturation of OLs after hypoxia, which was indicated as a remark decrease in the percentage of MBP+ out of Olig2+ cells (Fig. 3b, e). MFA and CBX treatment could rescue the reduction of MBP+/Olig2+ cell ratio (Fig. 3b, e).
OPC maturation was suppressed under hypoxic conditions, while Cx43 inhibitors rescued OPC differentiation. a, d After 1 day of hypoxia, OPC proliferation is increased compared to control according to the percentage of EdU+Olig2+ (EdU (red), Olig2 (green)). b, e Based on MBP (red, OL) and Olig2 staining (green, oligodendroglia lineage cells), the proportion of maturing oligodendrocyte decreased after 7 days of hypoxia compared to the control. In addition, Cx43 inhibitors decreased the proliferation of OPCs (a, d) but promoted OPCs differentiation (b, e). This was confirmed by Western blotting with PDGFR-α (OPCs marker) and MBP (maturing OL marker); β-actin acted as a loading control (c, f, g). Scale bars, 50 μm (a); 100 μm (b). Data are mean ± SD, *p < 0.05, ***p < 0.001, CoCl2 group vs control group by one-way ANOVA; #p < 0.05, ##p < 0.01, ###p < 0.001, MFA or CBX group vs CoCl2 group by one-way ANOVA
In agreement with immunofluorescent staining, Western blot confirmed the hypoxia-induced upregulation of PDGFR-α (OPCs marker) was inhibited by MFA or CBX treatment (Fig. 3c, f). Meanwhile, the hypoxia-induced MBP (mature OL marker) reduction could also be rescued by MFA and CBX treatment (Fig. 3c, g). Taken together, the results indicated that Cx43 inhibitors could suppress proliferation but promote differentiation of OPCs under mild chronic hypoxia.
Cx43 Inhibitors Suppressed Cx43 Hemichannel Activity and Glutamate Release Under Chronic Hypoxia
To establish the effect of Cx43 inhibitors on hemichannel activity, EtBr dye uptake was measured as previously reported (Giaume et al. 2012). Astrocyte showed that a significant increased dye uptake in hypoxia condition compared to normoxia (Fig. 4a, b). However, when incubated with Cx43 inhibitors, the hypoxia-induced dye uptake increase was significantly reduced (Fig. 4a, b). The Cx43 hemichannels are well-known to be a pathway to release glutamate (Ye et al. 2003). We found that, after 3 days of hypoxia treatment, the extracellular glutamate level was significantly higher than that under normoxic conditions (43.63 ± 4.87 versus 9.92 ± 5.84 μmol L−1, p < 0.001; Fig. 4c). At days 5 and 7 of hypoxia, the extracellular glutamate levels were relatively lower than earlier time point but remained higher than that under normoxia (28.65 ± 3.15 versus 14.00 ± 6.81 μmol L−1 at day 5; 27.09 ± 2.47 versus 13.30 ± 5.61 μmol L−1 at day 7; p < 0.05; Fig. 4c). MFA or CBX treatment significantly attenuated the glutamate release under chronic hypoxia at all time point (p < 0.05; Fig. 4c).
Cx43 hemichannel activity was upregulated under hypoxia. a, b Hypoxia could increase EtBr uptake in co-cultures, while MFA and CBX attenuated this effect. c Extracellular glutamate levels were increased during hypoxia, while MFA and CBX could markedly suppress this effect. Scale bar, 50 μm. Data are mean ± SD, *p < 0.05, ***p < 0.001, CoCl2 group vs control group by one-way ANOVA, #p < 0.05, ##p < 0.01, ###p < 0.001, MFA or CBX group vs CoCl2 group by one-way ANOVA
AMPA Receptor Inhibitor Partially Rescued the Limited OPC Maturation Under Hypoxia
Glutamate receptors are expressed in oligodendroglia lineage cells (De Biase et al. 2010; Micu et al. 2006) and have been shown to mediate excitotoxicity, migration, proliferation, and maturation of oligodendroglia lineage cells (Deng et al. 2003, 2006; Dohare et al. 2016; Li et al. 2013; Micu et al. 2006). We applied α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) or N-methyl-D-aspartate (NMDA) glutamate receptor inhibitors to test if OPCs were responding to the extracellular glutamate released from Cx43 hemichannels under chronic hypoxia. AMPA receptor antagonist NBQX (10 μM) or NMDA receptor antagonist DL-AP-5 (50 μM) could significantly decrease the percentage of EdU+Olig2+/Olig2+ proliferating OPCs (Fig. 5a, c). Interestingly, under hypoxia, NBQX but not DL-AP-5 treatment partially improved the percentage of MBP+/Olig2+ cells (Fig. 5b, d). These results indicated that AMPAR activation suppressed OPC maturation under hypoxia.
AMPAR antagonist promotes OPC maturation under hypoxic conditions. When cultures were treated with AMPAR and NMDAR antagonists (NBQX and AP-5, respectively) during hypoxia, both NBQX (10 μM) and AP-5 (50 μM) attenuated OPC proliferation (a, c). Only NBQX but not AP-5 partially rescue the limited differentiation of OPCs under hypoxia (b, d). Scale bar, 50 μm (a); 100 μm (b). Data are mean ± SD, *p < 0.05, **p < 0.01, CoCl2 or AP-5 group vs control group by one-way ANOVA; #p < 0.05, ##p < 0.01, NBQX or AP-5 group vs CoCl2 group by one-way ANOVA
Discussion
In present study, we showed that inhibition of astrocytic Cx43 could suppress glutamate release via hemichannels and promote OPC maturation after chronic hypoxia injury via AMPA receptor inhibition. In an astrocyte-OPC co-culture system, hypoxia could upregulate astrocytic Cx43 expression and induce astrocyte activation, as well as glutamate release via Cx43 hemichannels. Pharmacologically inhibiting astrocytic Cx43 could significantly suppress glutamate release and therefore improve differentiation of OPCs. Furthermore, AMPA receptor but not NMDA receptor antagonist partially enhanced the maturation of OPCs under hypoxia. This study has provided new insights into the role of astrocytic Cx43 involved in OPC differentiation.
OPC proliferation and differentiation after demyelination are mediated by multiple factors, including intercellular communication (Shindo et al. 2016) and extracellular environment (Rosenzweig and Carmichael 2015). Particularly, it has been known that astrocytes regulate oligodendrogenesis by secreting trophic factors into extracellular space, such as erythropoietin under hypoxic and reoxygenation injury in vitro (Kato et al. 2011) or brain-derived neurotrophic factor (BDNF) in the cuprizone-induced demyelination model (Fulmer et al. 2014). In addition, direct astrocyte and oligodendrocyte communication via gap junctions are essential for the proper functioning of myelination (Orthmann-Murphy et al. 2008). Furthermore, our group has found that inhibition of Cx43 channels could be protected for myelin sheath damage in chronic cerebral hypoperfusion mice model (unpublished data). Our study showed that astrocytic Cx43 hemichannels could release glutamate which resulted in AMPA receptor-mediated inhibition of OPC maturation after prolonged hypoxia in vitro.
Gap junctions are involved in maintaining myelin homeostasis. Astrocyte primarily expresses Cx43 and Cx30, whereas oligodendrocytes express Cx47, Cx32, and Cx29 (Orthmann-Murphy et al. 2008). Double knockout of Cx32 and Cx43 could give rise to white matter vacuolation and progressive loss of astrocytes and induce sensorimotor impairment, seizure activity, and early mortality (Magnotti et al. 2011). Mice lacking both astrocytic Cx30 and 43 exhibited myelin degeneration and vacuole formation while deletion either of these connexins could retain myelin integrity (Cotrina and Nedergaard 2012; Lutz et al. 2009). Deletion of astrocytic Cx43 affected OPC proliferation due to altered glucose support from astrocytes (Niu et al. 2016). Furthermore, peptide5, which blocks the Cx43 hemichannels, was shown to improve oligodendrocyte survival after brain ischemia (Davidson et al. 2012, 2013). Our results indicated that the inhibition of astrocytic Cx43 could be beneficial for facilitating OPC maturation after chronic hypoxia.
Cx43 hemichannels act as important pathway for release of glutamate, ATP, cytokines, playing an active role in neuroinflammation (Rouach et al. 2008). Accumulating evidence indicates a key role for glutamate signaling in white matter pathology (Butt et al. 2014). Glutamate-evoked Ca2+ signals in oligodendrocytes are closely related with OL differentiation and myelination, although the expression of AMPARs and NMDARs on oligodendroglia lineage cells is still undefined (De Biase et al. 2010; Li et al. 2013). It is thought that AMPARs were mainly expressed on OPCs and were downregulated in immature OL and OL. NMDARs, on the other hand, are believed to be restricted to immature OL and OL with generally low expressions, but absent in OPCs. White matter OPCs expressing AMPARs can be activated by glutamate (Hamilton et al. 2010). Glutamate released from axons could promote myelination of OL lineage cells during postnatal development (Kougioumtzidou et al. 2017). However, AMPAR activation in oligodendrocytes has also been shown to cause cell death and inhibit OPC maturation as well as remyelination in hypoxia-ischemia models (Follett et al. 2004; Volpe 2009). Meanwhile, it has been reported that NMDAR signaling was not required for OPC development (De Biase et al. 2011). Our data suggested that administration of an AMPAR but not NMDAR antagonist reduced OPC proliferation and promoted their differentiation into OLs.
In summary, astrocytic Cx43 hemichannels could result in increase in glutamate release after chronic hypoxia, which could impair OPC maturation and remyelination processes. Glutamate released via Cx43 hemichannels suppressed OPC differentiation partially mediated by AMPAR activation (Fig. 6). Application Cx43 inhibitors could rescue hypoxia-induced suppression of OPC differentiation. Based on our findings, astrocytic Cx43 hemichannels could potentially be a therapeutic target for facilitating OPC remyelination in hypoxia-induced white matter injury.
References
Butt AM, Fern RF, Matute C (2014) Neurotransmitter signaling in white matter. Glia 62:1762–1779. https://doi.org/10.1002/glia.22674
Cotrina ML, Nedergaard M (2012) Brain connexins in demyelinating diseases: therapeutic potential of glial targets. Brain Res 1487:61–68. https://doi.org/10.1016/j.brainres.2012.07.003
Davidson JO, Green CR, Nicholson LF, O'Carroll SJ, Fraser M, Bennet L, Gunn AJ (2012) Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol 71:121–132. https://doi.org/10.1002/ana.22654
Davidson JO, Green CR, Nicholson LF, Bennet L, Gunn AJ (2013) Connexin hemichannel blockade is neuroprotective after, but not during, global cerebral ischemia in near-term fetal sheep. Exp Neurol 248:301–308. https://doi.org/10.1016/j.expneurol.2013.06.026
De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM (2012) Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev Neurobiol 72:713–728. https://doi.org/10.1002/dneu.20976
De Biase LM, Nishiyama A, Bergles DE (2010) Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci 30:3600–3611. https://doi.org/10.1523/JNEUROSCI.6000-09.2010
De Biase LM et al (2011) NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci 31:12650–12662. https://doi.org/10.1523/jneurosci.2455-11.2011
Deng W, Rosenberg PA, Volpe JJ, Jensen FE (2003) Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc Natl Acad Sci USA 100:6801–6806. https://doi.org/10.1073/pnas.1136624100
Deng W, Neve RL, Rosenberg PA, Volpe JJ, Jensen FE (2006) Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit composition and cAMP-response element-binding protein regulate oligodendrocyte excitotoxicity. J Biol Chem 281:36004–36011. https://doi.org/10.1074/jbc.M606459200
Dohare P, Zia MT, Ahmed E, Ahmed A, Yadala V, Schober AL, Ortega JA, Kayton R, Ungvari Z, Mongin AA, Ballabh P (2016) AMPA-Kainate receptor inhibition promotes neurologic recovery in premature rabbits with intraventricular hemorrhage. J Neurosci 36:3363–3377. https://doi.org/10.1523/jneurosci.4329-15.2016
Follett PL, Deng W, Dai W, Talos DM, Massillon LJ, Rosenberg PA, Volpe JJ, Jensen FE (2004) Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci 24:4412–4420. https://doi.org/10.1523/JNEUROSCI.0477-04.2004
Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF (2014) Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J Neurosci 34:8186–8196. https://doi.org/10.1523/jneurosci.4267-13.2014
Giaume C, Orellana JA, Abudara V, Saez JC (2012) Connexin-based channels in astrocytes: how to study their properties. Methods Mol Biol 814:283–303. https://doi.org/10.1007/978-1-61779-452-0_19
Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait-Oumesmar B, Baron-van Evercooren A, Lachapelle F (2005) Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci 25:7924–7933. https://doi.org/10.1523/jneurosci.4890-04.2005
Gudz TI, Komuro H, Macklin WB (2006) Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci 26:2458–2466. https://doi.org/10.1523/jneurosci.4054-05.2006
Hamilton N, Vayro S, Wigley R, Butt AM (2010) Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia 58:66–79. https://doi.org/10.1002/glia.20902
Kato S, Aoyama M, Kakita H, Hida H, Kato I, Ito T, Goto T, Hussein MH, Sawamoto K, Togari H, Asai K (2011) Endogenous erythropoietin from astrocyte protects the oligodendrocyte precursor cell against hypoxic and reoxygenation injury. J Neurosci Res 89:1566–1574. https://doi.org/10.1002/jnr.22702
Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, Monyer H, Attwell D, Richardson WD (2017) Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6:e28080. https://doi.org/10.7554/eLife.28080.001
Li C, Xiao L, Liu X, Yang W, Shen W, Hu C, Yang G, He C (2013) A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61:732–749. https://doi.org/10.1002/glia.22469
Li X, Zhao H, Tan X, Kostrzewa RM, du G, Chen Y, Zhu J, Miao Z, Yu H, Kong J, Xu X (2015) Inhibition of connexin43 improves functional recovery after ischemic brain injury in neonatal rats. Glia 63:1553–1567. https://doi.org/10.1002/glia.22826
Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF (2009) Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci 29:7743–7752. https://doi.org/10.1523/jneurosci.0341-09.2009
Magnotti LM, Goodenough DA, Paul DL (2011) Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia 59:1064–1074. https://doi.org/10.1002/glia.21179
McTigue DM, Horner PJ, Stokes BT, Gage FH (1998) Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci 18:5354–5365
Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK (2006) NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439:988–992. https://doi.org/10.1038/nature04474
Miyamoto N, Pham LDD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, Ayata C, Kim KW, Boas D, Lo EH, Arai K (2013) Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 44:2573–2578. https://doi.org/10.1161/STROKEAHA.113.001530
Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K (2015) Astrocytes promote Oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci 35:14002–14008. https://doi.org/10.1523/jneurosci.1592-15.2015
Murtie JC, Zhou YX, Le TQ, Vana AC, Armstrong RC (2005) PDGF and FGF2 pathways regulate distinct oligodendrocyte lineage responses in experimental demyelination with spontaneous remyelination. Neurobiol Dis 19:171–182. https://doi.org/10.1016/j.nbd.2004.12.006
Niu J, Wang L, Liu S, Li C, Kong J, Shen HY, Xiao L (2012) An efficient and economical culture approach for the enrichment of purified oligodendrocyte progenitor cells. J Neurosci Methods 209:241–249. https://doi.org/10.1016/j.jneumeth.2012.05.032
Niu J, Li T, Yi C, Huang N, Koulakoff A, Weng C, Li C, Zhao CJ, Giaume C, Xiao L (2016) Connexin-based channels contribute to metabolic pathways in the oligodendroglial lineage. J Cell Sci 129:1902–1914. https://doi.org/10.1242/jcs.178731
Orthmann-Murphy JL, Abrams CK, Scherer SS (2008) Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci 35:101–116. https://doi.org/10.1007/s12031-007-9027-5
Rosenzweig S, Carmichael ST (2015) The axon-glia unit in white matter stroke: mechanisms of damage and recovery. Brain Res 1623:123–134. https://doi.org/10.1016/j.brainres.2015.02.019
Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, Giaume C (2002) Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell 94:457–475
Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C (2008) Astroglial metabolic networks sustain hippocampal synaptic transmission. Science (New York, NY) 322:1551–1555. https://doi.org/10.1126/science.1164022
Rovegno M, Soto PA, Saez PJ, Naus CC, Saez JC, von Bernhardi R (2015) Connexin43 hemichannels mediate secondary cellular damage spread from the trauma zone to distal zones in astrocyte monolayers. Glia 63:1185–1199. https://doi.org/10.1002/glia.22808
Rowitch DH, Kriegstein AR (2010) Developmental genetics of vertebrate glial-cell specification. Nature 468:214–222. https://doi.org/10.1038/nature09611
Schulz R, Gorge PM, Gorbe A, Ferdinandy P, Lampe PD, Leybaert L (2015) Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Ther 153:90–106. https://doi.org/10.1016/j.pharmthera.2015.06.005
Shindo A, Liang AC, Maki T, Miyamoto N, Tomimoto H, Lo EH, Arai K (2016) Subcortical ischemic vascular disease: roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab 36:187–198. https://doi.org/10.1038/jcbfm.2015.80
Tress O, Maglione M, May D, Pivneva T, Richter N, Seyfarth J, Binder S, Zlomuzica A, Seifert G, Theis M, Dere E, Kettenmann H, Willecke K (2012) Panglial gap junctional communication is essential for maintenance of myelin in the CNS. J Neurosci 32:7499–7518. https://doi.org/10.1523/jneurosci.0392-12.2012
Volpe JJ (2009) The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol 16:167–178. https://doi.org/10.1016/j.spen.2009.09.005
Wake H, Lee PR, Fields RD (2011) Control of local protein synthesis and initial events in myelination by action potentials. Science (New York, NY) 333:1647–1651. https://doi.org/10.1126/science.1206998
Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ (2004) Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci 25:252–262. https://doi.org/10.1016/j.mcn.2003.10.014
Wu X, Liu Y, Chen X, Sun Q, Tang R, Wang W, Yu Z, Xie M (2013) Involvement of TREK-1 activity in astrocyte function and neuroprotection under simulated ischemia conditions. J Mol Neurosci 49:499–506. https://doi.org/10.1007/s12031-012-9875-5
Xie M, Yi C, Luo X, Xu S, Yu Z, Tang Y, Zhu W, Du Y, Jia L, Zhang Q, Dong Q, Zhu W, Zhang X, Bu B, Wang W (2011) Glial gap junctional communication involvement in hippocampal damage after middle cerebral artery occlusion. Ann Neurol 70:121–132. https://doi.org/10.1002/ana.22386
Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23:3588–3596
Yi C, Ezan P, Fernandez P, Schmitt J, Saez JC, Giaume C, Koulakoff A (2017) Inhibition of glial hemichannels by boldine treatment reduces neuronal suffering in a murine model of Alzheimer's disease. Glia 65:1607–1625. https://doi.org/10.1002/glia.23182
Zonouzi M, Renzi M, Farrant M, Cull-Candy SG (2011) Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells. Nat Neurosci 14:1430–1438. https://doi.org/10.1038/nn.2942
Acknowledgments
The investigation was supported by the National Natural Science Foundation of China (81571113, 61327902), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_14R20), and grant from the Fundamental Research Funds for the Central Universities (2017KFYXJJ097), the Huazhong University of Science and Technology “Double Top” Construction Pro-ject of International Cooperation (grant 540-5001540013). Acknowledgements to Jia Li, Li Xu, and Xiaojiang Huang in contribution in preliminary data collections.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOC 365 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, Q., Wang, Z., Tian, Y. et al. Inhibition of Astrocyte Connexin 43 Channels Facilitates the Differentiation of Oligodendrocyte Precursor Cells Under Hypoxic Conditions In Vitro. J Mol Neurosci 64, 591–600 (2018). https://doi.org/10.1007/s12031-018-1061-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1061-y