Abstract
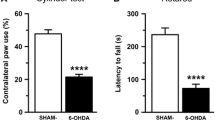
Parkinson’s disease is the second most common neurodegenerative disorder and remains incurable. Many potential compensatory mechanisms have now been proposed; these are both dopaminergic, focused on enhancing effects or exposure to existing dopamine, and non-dopaminergic, being focused on reducing activity of the indirect striatal output pathway. In the present study, the effects of serotonin, gamma-aminobutyric acid, and bone marrow cell supplementation intranigrally to the substantia nigra on unilateral 6-hydroxydopamine-infused rats were analyzed individually. Dopaminergic binding parameters were done by Scatchard analysis of dopamine D1 receptor-binding assay using [3H]SCH 23390. In the corpus striatum, 6-hydroxydopamine-infused rats showed a significant decrease in B max (P < 0.001), and in cerebral cortex, they showed a significant increase in B max (P < 0.001) compared to control. Real-time polymerase chain reaction amplification of dopamine D1 was downregulated (P < 0.001) in the corpus striatum of 6-hydroxydopamine-infused rats compared to control, whereas in the cerebral cortex, it showed a significant upregulation (P < 0.001). Behavioral studies were carried out to confirm the biochemical and molecular studies. Serotonin and gamma-aminobutyric acid supplementation reversed these changes to control. The bone marrow cell-treated group of our studies does not show much significant change as compared to the serotonin and gamma-aminobutyric acid-supplemented groups. The alterations in dopamine D1 receptor-binding parameters and gene expression during Parkinson’s model were reversed by serotonin and gamma-aminobutyric acid supplementation in our experiments, which has clinical significance in the management of the disease.





Similar content being viewed by others
References
Andersson, C., Tytell, M., & Brunso-Bechtold, J. (1993). Transplantation of cultured type 1 astrocyte cell suspensions into young, adult and aged rat cortex: Cell migration and survival. International Journal of Developmental Neuroscience, 11(5), 555–568.
Andrew, R., Watson, D. G., Best, S. A., Midgley, J. M., Wenlong, H., & Petty, R. K. H. (1993). The determination of hydroxydopamines and other trace amines in the urine of Parkinsonian patients and normal controls. Neurochemical Research, 18(11), 1175–1177.
Atkins, P. T., Surmeier, D. J., & Kitai, S. T. (1990). Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature, 344, 240–242.
Azizi, S. A., Stokes, D., Augelli, B. J., Digirolamo, C., & Prockop, D. J. (1998). Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—Similarities to astrocyte grafts. Proceedings of the National Academy of Sciences of the United States of America, 95, 3908–3913.
Biju, M. P., Pyroja, S., Rajeshkumar, N. V., & Paulose, C. S. (2002). Enhanced GABA(B) receptor in neoplastic rat liver: Induction of DNA synthesis by baclofen in hepatocyte cultures. Journal of Biochemistry, Molecular Biology and Biophysics, 6(3), 209–14.
Bove, J., Prou, D., Perier, C., & Przedborski, S. (2005). Toxin-induced models of Parkinson’s disease. NeuroRx, 2(3), 484–494.
Caterson, E. J., Nesti, L. J., Danielson, K., & Tuan, R. S. (2002). Human marrow-derived mesenchymal progenitor cells: Isolation, culture expansion, and analysis of differentiation. Molecular Biotechnology, 20(3), 245–256.
Dohlman, H. G., Thorner, J., Caron, M. G., & Lefkowitz, R. J. (1991). Model systems for the study of seven-transmembrane-segment receptors. Annual Review of Biochemistry, 60, 653–688.
Faull, R. L., & Laverty, R. (1969). Changes in dopamine levels in the corpus striatum following lesions in the substantia nigra. Experimental Neurology, 23, 332–340.
Ferré, S., O’Connor, W. T., Fuxe, K., & Ungerstedt, U. (1993). The striopallidal neuron: a main locus for adenosine–dopamine interactions in the brain. Journal of Neuroscience, 13(12), 5402–6.
Glowinski, J., & Iversen, L. L. (1966). Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. Journal of Neurochemistry, 13(8), 655–659.
Hamani, C., Saint-Cyr, J. A., Fraser, J., Kaplitt, M., & Lozano, A. M. (2004). The subthalamic nucleus in the context of movement disorders. Brain, 127, 4–20.
Hellmann, M. A., Panet, H., Barhum, Y., Melamed, E., & Offen, D. (2005). Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neuroscience Letters, 395(2), 124–8.
Hely, M. A., Morris, J. G., Reid, W. G., et al. (1994). The Sydney multicentre study of Parkinson’s disease: A randomised, prospective 5 years study comparing lowdose bromocriptine with lowdose levodopa-carbidopa. Journal of Neurology, Neurosurgery and Psychiatry, 57(8), 903–910.
Herve, D., Pickel, V. M., Joh, T. H., & Beaudet, A. (1987). Serotonin axon terminals in the ventral tegmental area of the rat: Fine structure and synaptic input to dopaminergic neurons. Brain Research, 435, 71–83.
Jeon, B. S., Jackson-Lewis, V., & Burke, R. E. (1995). 6-Hydroxydopamine lesion of the rat substantia nigra: Time course and morphology of cell death. Neurodegeneration, 4(2), 131–137.
Joseph, A., Antony, S., & Paulose, C. S. (2008). Increased glutamate receptor gene expression in the cerebral cortex of insulin induced hypoglycemic and streptozotocin-induced diabetic rats. Neuroscience, 156, 298–304.
Kawaguchi, Y., Wilson, C. J., Augood, S. J., & Emson, P. C. (1995). Striatal interneurones: Chemical, physiological and morphological characterization. Trends in Neurosciences, 18(12), 527–535.
Kirk, I. P., & Richardson, P. J. (1994). Adenosine A2a receptor-mediated modulation of striatal [3H]GABA and [3H]acetylcholine release. Journal of Neurochemistry, 62(3), 960–966.
Kurokawa, M., Koga, K., Kase, H., Nakamura, J., & Kuwana, Y. (1996). Adenosine A2a receptor-mediated modulation of striatal acetylcholine release in vivo. Journal of Neurochemistry, 66(5), 1882–1888.
Lang, A. E. (2009). When and how should treatment be started in Parkinson disease? Neurology, 72(7), S39–43.
Levy, Y. S., Bahat-Stroomza, M., Barzilay, R., et al. (2008). Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson’s disease. Cytotherapy, 10(4), 340–52.
Lowry, O. H., Rosenbrough, N. H., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Majumdar, M. K., Thiede, M. A., Mosca, J. D., Moorman, M., & Gerson, S. L. (1998). Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. Journal of Cell Physiology, 176(1), 57–66.
Margaret, A. G., Katja, B., Glenn, P., Conner, A. S., & Richard, C. M. (1996). Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. Journal of Experimental Medicine, 183(4), 1797–1806.
Mizoguchi, K., Yuzurihara, M., Ishige, A., Sasaki, H., Chui, D. H., & Tabira, T. (2000). Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. Journal of Neuroscience, 20(4), 1568–1574.
Morelli, M., Fenu, S., Pinna, A., & Di Chiara, G. (1994). Adenosine A2 receptors interact negatively with dopamine D1 and D2 receptors in unilaterally 6-hydroxydopamine-lesioned rats. European Journal of Pharmacology, 251(1), 21–25.
Mori, A., Shindou, T., Ichimura, M., Nonaka, H., & Kase, H. (1996). The role of adenosine A2a receptors in regulating GABAergic synaptic transmission in striatal medium spiny neurons. Journal of Neuroscience, 16(2), 605–611.
Munoz, E. G., Woodbury, D., & Black, I. (2003). Marrow stromal cells, mitosis, and neuronal differentiation: Stem cell and precursor functions. Stem Cells, 21(4), 437–448.
Muñoz, A., Li, Q., Gardoni, F., et al. (2008). Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain, 131, 3380–94.
Nedergaard, S., Bolam, J. P., & Greenfield, S. A. (1988). Facilitation of a dendritic calcium conductance by 5-hydroxytryptamine in the substantia nigra. Nature, 333, 174–177.
Nicholson, S. L., & Brotchie, J. M. (2002). 5-hydroxytryptamine (5-HT, serotonin) and Parkinson’s disease—Opportunities for novel therapeutics to reduce the problems of levodopa therapy. European Journal of Neurology, 9(3), 1–6.
Paulose, C. S., & Savitha, B. (2008). Adrenergic, dopaminergic and serotonergic gene expression 3 in low dose, long time insulin and somatotropin treatment to ageing rats: Rejuvenation of brain function. Biogerontology, 9, 429–439. doi:10.1007/s10522-008-9183-1.
Paxinos, G., & Watson, C. (1998). The rat brain in stereotaxic coordinates (p. 105). Burlington: Elsevier.
Poewe, W. (2009). Treaents for Parkinson disease—Past achievements and current clinical needs. Neurology, 72, S65–S73.
Precht, W., & Yoshida, M. (1971). Blockage of caudate-evoked inhibition of neurons in the substantia nigra by picrotoxin. Brain Research, 32(1), 229–233.
Rafii, S., Shapiro, F., Rimarachin, J., et al. (1994). Isolation and characterization of human bone marrow microvascular endothelial cells: Hematopoietic progenitor cell adhesion. Blood, 84(1), 10–19.
Sadan, O., Bahat-Stromza, M., Barhum, Y., et al. (2009). Protective effects of neurotrophic factors secreting cells in a 6OHDA rat model of Parkinson disease. Stem Cells. doi:10.1089/scd.2008.0411.
Sajadi, A., Bensadoun, J. C., Schneider, B. L., Lo Bianco, C., & Aebischer, P. (2006). Transient striatal delivery of GDNF via encapsulated cells leads to sustained behavioral improvement in a bilateral model of Parkinson’s disease. Neurobiology of Disease, 22(1), 119–129.
Scatchard, G. (1949). The attractions of proteins for small molecules and ions. Annals of the New York Academy of Sciences, 51(4), 660–672.
Schallert, T., & Woodlee, M. T. (2004). The interplay between behavior and neurodegeneration in rat models of Parkinson's disease and stroke. Restorative Neurology and Neuroscience, 22(3–5), 153–161.
Schallert, T., Fleming, S. M., Leasure, J. L., Tillerson, J. L., & Bland, S. T. (2000). CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology, 39(5), 777–787.
Sudha, B., & Paulose, C. S. (1988). Induction of DNA synthesis in primary cultures of rat hepatocytes by serotonin: Possible involvement of serotonin S2 receptor. Hepatology, 27(1), 62–66.
Suon, S., Jin, H., Donaldson, A. E., et al. (2004). Transient differentiation of adult human bone marrow cells into neuron-like cells in culture: Development of morphological and biochemical traits is mediated by different molecular mechanisms. Stem Cells and Development, 13(6), 625–635.
Ungerstedt, U. (1968). 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. European Journal of Pharmacology, 5, 107–110.
Ungerstedt, U. (1971). Postsynaptic supersensitivity after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiologica Scandinavica. Supplementum, 367, 95–122.
Wang, J., & Johnson, K. M. (1995). Regulation of striatal cyclic-3′, 5′-adenosine monophosphate accumulation and GABA release by glutamate metabotropic and dopamine D1 receptors. Journal of Pharmacology and Experimental Therapeutics, 275(2), 877–884.
Wichmann, T., & DeLong, M. R. (2003). Pathophysiology of Parkinson’s disease: The MPTP primate model of the human disorder. Annals of the New York Academy of Sciences, 991, 199–213.
Zhou, H. F., & Lund, R. D. (1992). Migration of astrocytes transplanted to the midbrain of neonatal rats. Journal of Comparative Neurology, 317(2), 145–155.
Acknowledgments
This work was supported by research grants from DBT, DST, ICMR, Govt. of India and KSCSTE, Govt. of Kerala to Dr. C. S. Paulose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nandhu, M.S., Fabia, E.T. & Paulose, C.S. Dopamine D1 Receptor Gene Expression Studies in Unilateral 6-Hydroxydopamine-Lesioned Parkinson’s Rat: Effect of 5-HT, GABA, and Bone Marrow Cell Supplementation. J Mol Neurosci 41, 1–11 (2010). https://doi.org/10.1007/s12031-009-9213-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-009-9213-8