Abstract
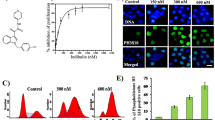
A method was developed to assess mitochondrial movement in the living cell that is dependent, in part, on microtubule and/or associating protein interactions. The leader sequence from cytochrome-c was used to drive DsRed2 fluorescent proteins to accumulate in the mitochondria, thus enabling to follow mitochondrial (cytochrome-c’s) movement. For calculating the percentage of mitochondrial movement, an image-processing program was used (ImageJ). Paclitaxel, an antitumor agent, is a potent microtubule-stabilizing agent that increases the stability of tubulin polymers, inhibiting mitosis and mitochondrial activity in dividing cells. Here, we tested whether paclitaxel inhibits mitochondrial movement in pheochromocytoma cells (a neuronal model, when tested in a differentiated state). While a 2-day exposure to paclitaxel resulted in cellular toxicity (measured as inhibition of mitochondrial activity), 2–3 h exposure to paclitaxel were sufficient to inhibit mitochondrial movement as assessed in 10–20-s imaging sessions in living cells. Mitotracker deep-red staining validated the staining obtained with DsRed2-cytochrome-c and identified intact mitochondria. Results showed a significant paclitaxel dose-dependent inhibition of mitochondrial movement. This new method should enable further assessment of microtubule-interacting drugs and other cytoskeletal components for their potential influence of mitochondrial movement as a test for activity and side effects.






Similar content being viewed by others
References
Abramoff, M. D., Magelhaes, P. J., & Ram, S. J. (2004). Image processing with ImageJ. Biophotonics International, 11, 36–42.
Allen, R. D., Metuzals, J., Tasaki, I., Brady, S. T., & Gilbert, S. P. (1982). Fast axonal transport in squid giant axon. Science, 218, 1127–1129.
André, N., Braguer, D., Brasseur, G., Gonçalves, A., Lemesle-Meunier, D., Guise, S., et al. (2000). Paclitaxel induces release of cytochrome c from mitochondria isolated from human neuroblastoma cells’. Cancer Res, 1, 5349–5353.
Arendt, T. (2003). Synaptic plasticity and cell cycle activation in neurons are alternative effector pathways: the ‘Dr. Jekyll and Mr. Hyde concept’ of Alzheimer’s disease or the yin and yang of neuroplasticity. Prog Neurobiol, 71, 83–248.
Baas, P. W., & Qiang, L. (2005). Neuronal microtubules: when the MAP is the roadblock. Trends Cell Biology, 15, 183–187.
Baird, G. S., Zacharias, D. A., & Tsien, R. Y. (2000). Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proceedings of the National Academy of Sciences of the United States of America, 97, 11984–11989.
Bassan, M., Zamostiano, R., Davidson, A., Pinhasov, A., Giladi, E., Perl, O., et al. (1999). Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. Journal of Neurochemistry, 72, 1283–1293.
Carré, M., André, N., Carles, G., Borghi, H., Brichese, L., Briand, C., et al. (2002). Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. Journal of Biological Chemistry, 13, 33664–33669.
Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W., & Prasher, D. C. (1994). Green fluorescent protein as a marker for gene expression. Science, 263, 802–805.
Coleman, M. (2005). Axon degeneration mechanisms: commonality amid diversity. Nature Reviews Neuroscience, 6, 889–898.
Desai, A., & Mitchison, T. J. (1997). Microtubule polymerization dynamics. Annual Review of Cell and Developmental Biology, 13, 83–117.
Ebneth, A., Godemann, R., Stamer, K., Illenberger, S., Trinczek, B., & Mandelkow, E. (1998). Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. Journal of Cell Biology, 143, 777–794.
Evtodienko, Y. V., Teplova, V. V., Sidash, S. S., Ichas, F., & Mazat, J. P. (1996). Microtubule-active drugs suppress the closure of the permeability transition pore in tumour mitochondria. FEBS Letters, 9, 86–88.
Flatters, S. J., & Bennett, G. J. (2006). Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain, 122, 245–257.
Gotz, J., Schild, A., Hoerndli, F., & Pennanen, L. (2004). Amyloid-induced neurofibrillary tangle formation in Alzheimer’s disease: insight from transgenic mouse and tissue-culture models. International Journal of Developmental Neuroscience, 22, 453–465.
Gozes, I., & Littauer, U. Z. (1978). Tubulin microheterogeneity increases with rat brain maturation. Nature, 276, 411–413.
Gozes, I., & Littauer, U. Z. (1982). Microtubule protein: tubulin. Scandinavian Journal of Immunology Supplement, 9, 299–316.
Gozes, I., & Sweadner, K. J. (1981). Multiple tubulin forms are expressed by a single neurone. Nature, 294, 477–480.
Greene, L. A., & Tischler, A. S. (1976). Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proceedings of the National Academy of Sciences of the United States of America, 73, 2424–2428.
Hafezparast, M., Klocke, R., Ruhrberg, C., Marquardt, A., Ahmad-Annuar, A., Bowen, S., et al. (2003). Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science, 300, 808–812.
Jin, H. W., Flatters, S. J., Xiao, W. H., Mulhern, H. L., & Bennett, G. J. (2008). Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-l-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Experimental Neurology, 210, 229–237.
Jordan, M. A., & Wilson, L. (2004). Microtubules as a target for anticancer drugs. Nature Reviews Cancer, 4, 253–265.
Karbowski, M., Spodnik, J. H., Teranishi, M., Wozniak, M., Nishizawa, Y., Usukura, J., et al. (2001). Opposite effects of microtubule-stabilizing and microtubule-destabilizing drugs on biogenesis of mitochondria in mammalian cells. Journal of Cell Science, 114, 281–291.
Kidd, J. F., Pilkington, M. F., Schell, M. J., Fogarty, K. E., Skepper, J. N., Taylor, C. W., et al. (2002). Paclitaxel affects cytosolic calcium signals by opening the mitochondrial permeability transition pore. Journal of Biological Chemistry, 22, 6504–6510.
Kingston, D. G., Hawkins, D. R., & Ovington, L. (1982). New taxanes from Taxus brevifolia. Journal of Natural Products, 45, 466–470.
Larroque, A. L., Dubois, J., Thoret, S., Aubert, G., Chiaroni, A., Guéritte, F., et al. (2007). Novel C2–C3′ N-peptide linked macrocyclic taxoids. Part 2: synthesis and biological activities of docetaxel analogues with a peptide side chain at C2 and their macrocyclic derivatives. Bioorganic and Medicinal Chemistry, 15, 563–574.
Luduena, R. F. (1998). Multiple forms of tubulin: different gene products and covalent modifications. International Review of Cytology, 178, 207–275.
Mandelkow, E. M., Thies, E., Trinczek, B., Biernat, J., & Mandelkow, E. (2004). MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. Journal of Cell Biology, 167, 99–110.
Manfredi, J. J., Parness, J., & Horwitz, S. B. (1982). Taxol binds to cellular microtubules. Journal of Cell Biology, 94, 688–696.
Morris, R. L., & Hollenbeck, P. J. (1993). The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. Journal of Cell Science, 104, 917–927.
Nakata, T., & Yorifuji, H. (1999). Morphological evidence of the inhibitory effect of taxol on the fast axonal transport. Neurosciences Research, 35, 113–122.
Orr, G. A., Verdier-Pinard, P., McDaid, H., & Horwitz, S. B. (2003). Mechanisms of Taxol resistance related to microtubules. Oncogene, 22, 7280–7295.
Parekh, H., & Simpkins, H. (1997). The transport and binding of taxol. General Pharmacology, 29, 167–172.
Ramalingam, S., & Belani, C. (2008). Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist, 13, 5–13, Review.
Reinecke, P., Corvin, J., Gabbert, H. E., & Gerharz, C. D. (1997). Antiproliferative effects of paclitaxel (Taxol) on human renal clear cell carcinomas in vitro. European Journal of Cancer, 33, 1122–1129.
Rizzuto, R., Brini, M., Pizzo, P., Murgia, M., & Pozzan, T. (1995). Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Current Biology, 5, 635–642.
Roytta, M., Horwitz, S. B., & Raine, C. S. (1984). Taxol-induced neuropathy: short-term effects of local injection. Journal of Neurocytology, 13, 685–701.
Schiff, P. B., & Horwitz, S. B. (1980). Taxol stabilizes microtubules in mouse fibroblast cells. Proceedings of the National Academy of Sciences of the United States of America, 77, 1561–1565.
Sciaky, N., Presley, J., Smith, C., Zaal, K. J., Cole, N., Moreira, J. E., et al. (1997). Golgi tubule traffic and the effects of brefeldin A visualized in living cells. Journal of Cell Biology, 139, 1137–1155.
Sharp, D. J., Rogers, G. C., & Scholey, J. M. (2000). Microtubule motors in mitosis. Nature, 407, 41–47.
Shemesh, O. A., Erez, H., Ginzburg, I., & Spira, M. E. (2008). Tau-induced traffic jams reflect organelles accumulation at points of microtubule polar mismatching. Traffic, 9, 458–471.
Shima, D. T., Haldar, K., Pepperkok, R., Watson, R., & Warren, G. (1997). Partitioning of the Golgi apparatus during mitosis in living HeLa cells. Journal of Cell Biology, 137, 1211–1228.
Stamer, K., Vogel, R., Thies, E., Mandelkow, E., & Mandelkow, E. M. (2002). Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. Journal of Cell Biology, 156, 1051–1063.
Vale, R. D., Schnapp, B. J., Reese, T. S., & Sheetz, M. P. (1985). Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell, 40, 449–454.
Varbiro, G., Veres, B., Gallyas, F. Jr., & Sumegi, B. (2001). Direct effect of Taxol on free radical formation and mitochondrial permeability transition. Free Radical Biology & Medicine, 15, 548–558.
Wacker, I., Kaether, C., Kromer, A., Migala, A., Almers, W., & Gerdes, H. H. (1997). Microtubule-dependent transport of secretory vesicles visualized in real time with a GFP-tagged secretory protein. Journal of Cell Science, 110(Pt 13), 1453–1463.
Wani, M. C., Taylor, H. L., Wall, M. E., Coggon, P., & McPhail, A. T. (1971). Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. Journal of the American Chemical Society, 93, 2325–2327.
Acknowledgements
This work is the fulfillment of the M.Sc. thesis requirements of Mr. Tal Shprung. We thank Dr. Yevgeny Berdichevsky for his help with the cloning techniques, Dr. Leonid Mittelman for his help with the imaging equipment and Drs. Alistair Stewart, Sharon Furman-Assaf, and Bruce Morimoto for critical reading of the manuscript and excellent suggestions. Support was provided by the BSF, ISF, and Allon Therapeutics Inc. Professor Gozes is the incumbent of the Lily and Avraham Gildor Chair for the Investigation of Growth Factors, and the Director of the Adams Super Center for Brain Studies, the Levie–Edersheim–Gitter Institute for Functional Brain Imaging and the Dr. Diana and Zelman Elton (Elbaum) Laboratory for Molecular Neuroendocrinology. Professor Illana Gozes serves as the Chief Scientific Officer of Allon Therapeutics Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shprung, T., Gozes, I. A Novel Method for Analyzing Mitochondrial Movement: Inhibition by Paclitaxel in a Pheochromocytoma Cell Model. J Mol Neurosci 37, 254–262 (2009). https://doi.org/10.1007/s12031-008-9129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9129-8