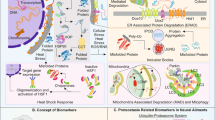
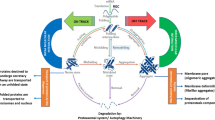
Abstract
Most neurodegenerative disorders are characterised by deposits of aggregated proteins that are readily visualised by light microscopy. Although the presence of such a bulky structure inside the cell or in the extracellular space is likely not to be healthy, over recent years the idea has emerged that these end-stage aggregates are a relatively safe way to deposit harmful aberrant proteins. Protein quality control is a multi-level security system to safeguard cells from aberrant proteins and is therefore a protective response. However, protein quality control may turn destructive in case of impairment of protein quality control for example by aging or because of overflow of the quality control systems due to prolonged exposure. In many cases the medicine is worse than the cause and the “protective” response of the cell to aggregates kills the cell, rather than the aggregate itself. Here we review the role of protein quality control in neurodegeneration and aim to distinguish protective and destructive responses to aggregates in order to find targets for therapeutic intervention.
Similar content being viewed by others
References
Anglade, P., Vyas, S., Javoy-Agid, F., Herrero, M. T., Michel, P. P., & Marquez, J., et al. (1997). Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histology and Histopathology, 12, 25–31.
Arvan, P., Zhao, X., Ramos-Castaneda, J., & Chang, A. (2002). Secretory pathway quality control operating in Golgi, plasmalemmal, and endosomal systems. Traffic, 3, 771–780.
Atkin, J. D., Farg, M. A., Turner, B. J., Tomas, D., Lysaght, J. A., & Nunan, J., et al. (2006). Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. Journal of Biological Chemistry, 281, 30152–30165.
Auld, K. L., & Silver, P. A. (2006). Transcriptional regulation by the proteasome as a mechanism for cellular protein homeostasis. Cell Cycle, 5, 1503–1505.
Bence, N. F., Sampat, R. M., & Kopito, R. R. (2001). Impairment of the ubiquitin-proteasome system by protein aggregation. Science, 292, 1552–1555.
Beranger, F., Mange, A., Goud, B., & Lehmann, S. (2002). Stimulation of PrP(C) retrograde transport toward the endoplasmic reticulum increases accumulation of PrP(Sc) in prion-infected cells. Journal of Biological Chemistry, 277, 38972–38977.
Bowman, A. B., Yoo, S. Y., Dantuma, N. P., & Zoghbi, H. Y. (2005). Neuronal dysfunction in a polyglutamine disease model occurs in the absence of ubiquitin-proteasome system impairment and inversely correlates with the degree of nuclear inclusion formation. Human Molecular Genetics, 14, 679–691.
Boyce, M., Bryant, K. F., Jousse, C., Long, K., Harding, H. P., & Scheuner, D., et al. (2005). A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science, 307, 935–939.
Campana, V., Sarnataro, D., & Zurzolo, C. (2005). The highways and byways of prion protein trafficking. Trends in Cell Biology, 15, 102–111.
Campana, V., Sarnataro, D., Fasano, C., Casanova, P., Paladino, S., & Zurzolo, C. (2006). Detergent-resistant membrane domains but not the proteasome are involved in the misfolding of a PrP mutant retained in the endoplasmic reticulum. Journal of Cell Science, 119, 433–442.
Chafekar, S. M., Hoozemans, J. J. M., Zwart, R., Baas, F., & Scheper, W. (2007). Aβ1-42 induces mild endoplasmic reticulum stress in an aggregation state dependent manner. Antioxidants & Redox Signalling. (in press).
Cooper, A. A., Gitler, A. D., Cashikar, A., Haynes, C. M., Hill, K. J., & Bhullar, B., et al. (2006). Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science, 313, 324–328.
De Pril, R., Fischer, D. F., Maat-Schieman, M. L., Hobo, B., de Vos, R. A., & Brunt, E. R., et al. (2004). Accumulation of aberrant ubiquitin induces aggregate formation and cell death in polyglutamine diseases. Human Molecular Genetics, 13, 1803–1813.
De Vrij, F. M., Fischer, D. F., van Leeuwen, F. W., & Hol, E. M. (2004). Protein quality control in Alzheimer’s disease by the ubiquitin proteasome system. Progress in Neurobiology, 74, 249–270.
Diaz-Hernandez, M., Moreno-Herrero, F., Gomez-Ramos, P., Moran, M. A., Ferrer, I., & Baro, A. M., et al. (2004). Biochemical, ultrastructural, and reversibility studies on huntingtin filaments isolated from mouse and human brain. Journal of Neuroscience, 24, 9361–9371.
Elsasser, S., & Finley, D. (2005). Delivery of ubiquitinated substrates to protein-unfolding machines. Nature Cell Biology, 7, 742–749.
Forman, M. S., Lee, V. M., & Trojanowski, J. Q. (2003). ‘Unfolding’ pathways in neurodegenerative disease. Trends in Neurosciences, 26, 407–410.
Friedlander, R., Jarosch, E., Urban, J., Volkwein, C., & Sommer, T. (2000). A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nature Cell Biology, 2, 379–384.
Gao, M., & Karin, M. (2005). Regulating the regulators: Control of protein ubiquitination and ubiquitin-like modifications by extracellular stimuli. Molecular Cell, 19, 581–593.
Gilch, S., Winklhofer, K. F., Groschup, M. H., Nunziante, M., Lucassen, R., & Spielhaupter, C., et al. (2001). Intracellular re-routing of prion protein prevents propagation of PrP(Sc) and delays onset of prion disease. EMBO Journal, 20, 3957–3966.
Girod, A., Storrie, B., Simpson, J. C., Johannes, L., Goud, B., & Roberts, L. M., et al. (1999). Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nature Cell Biology, 1, 423–430.
Goldberg, A. L. (2003). Protein degradation and protection against misfolded or damaged proteins. Nature, 426, 895–899.
Hamos, J. E., Oblas, B., Pulaski-Salo, D., Welch, W. J., Bole, D. G., & Drachman, D. A. (1991). Expression of heat shock proteins in Alzheimer’s disease. Neurology, 41, 345–350.
Hershko, A. (2005). The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture). Angewandte Chemie. International Edition in English, 44, 5932–5943.
Hetz, C., Russelakis-Carneiro, M., Maundrell, K., Castilla, J., & Soto, C. (2003). Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO Journal, 22, 5435–5445.
Hetz, C., Russelakis-Carneiro, M., Walchli, S., Carboni, S., Vial-Knecht, E., & Maundrell, K., et al. (2005). The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. Journal of Neuroscience, 25, 2793–2802.
Hicke, L., Schubert, H. L., & Hill, C. P. (2005). Ubiquitin-binding domains. Nature Reviews. Molecular Cell Biology, 6, 610–621.
Hitomi, J., Katayama, T., Eguchi, Y., Kudo, T., Taniguchi, M., & Koyama, Y., et al. (2004). Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. Journal of Cell Biology, 165, 347–356.
Hol, E. M., van Leeuwen, F. W., & Fischer, D. F. (2005). The proteasome in Alzheimer’s disease and Parkinson’s disease: Lessons from ubiquitin B+1. Trends in Molecular Medicine, 11, 488–495.
Hol, E. M., Fischer, D. F., Ovaa, H., & Scheper, W. (2006). Ubiquitin proteasome system as a pharmacological target in neurodegeneration. Expert Review of Neurotherapeutics, 6, 1337–1347.
Hong, M., Li, M., Mao, C., & Lee, A. S. (2004). Endoplasmic reticulum stress triggers an acute proteasome-dependent degradation of ATF6. Journal of Cellular Biochemistry, 92, 723–732.
Hoozemans, J. J., Veerhuis, R., Van Haastert, E. S., Rozemuller, J. M., Baas, F., & Eikelenboom, P., et al. (2005). The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathologica (Berlin), 110, 165–172.
Hoozemans, J. J. M., Van Haastert, E. S., Eikelenboom, P., De Vos, R. A. I., Rozemuller, J. M., & Scheper, W. (2007). Activation of the unfolded protein response in Parkinson’s disease. Biochemical and Biophysical Research Communications, 354, 707–711.
Imai, Y., Soda, M., Hatakeyama, S., Akagi, T., Hashikawa, T., & Nakayama, K. I., et al. (2002). CHIP is associated with Parkin, a gene responsible for familial Parkinson’s disease, and enhances its ubiquitin ligase activity. Molecular Cell, 10, 55–67.
Imai, Y., Soda, M., Inoue, H., Hattori, N., Mizuno, Y., & Takahashi, R. (2001). An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell, 105, 891–902.
Ironside, J. W., McCardle, L., Hayward, P. A., & Bell, J. E. (1993). Ubiquitin immunocytochemistry in human spongiform encephalopathies. Neuropathology & Applied Neurobiology, 19, 134–140.
Iwata, A., Christianson, J. C., Bucci, M., Ellerby, L. M., Nukina, N., & Forno, L. S., et al. (2005a). Increased susceptibility of cytoplasmic over nuclear polyglutamine aggregates to autophagic degradation. Proceedings of the National Academy of Sciences of the United States of America, 102, 13135–13140.
Iwata, A., Riley, B. E., Johnston, J. A., & Kopito, R. R. (2005b). HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. Journal of Biological Chemistry, 280, 40282.
Johnston, J. A., Ward, C. L., & Kopito, R. R. (1998). Aggresomes: A cellular response to misfolded proteins. Journal of Cell Biology, 143, 1883–1898.
Kanuka, H., Kuranaga, E., Hiratou, T., Igaki, T., Nelson, B., & Okano, H., et al. (2003). Cytosol-endoplasmic reticulum interplay by Sec61alpha translocon in polyglutamine-mediated neurotoxicity in Drosophila. Proceedings of the National Academy of Sciences of the United States of America, 100, 11723–11728.
Keller, J. N., Dimayuga, E., Chen, Q., Thorpe, J., Gee, J., & Ding, Q. (2004). Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. International Journal of Biochemistry & Cell Biology, 36, 2376–2391.
Kieran, D., Kalmar, B., Dick, J. R., Riddoch-Contreras, J., Burnstock, G., & Greensmith, L. (2004). Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nature Medicine, 10, 402–405.
Kiffin, R., Christian, C., Knecht, E., & Cuervo, A. M. (2004). Activation of chaperone-mediated autophagy during oxidative stress. Molecular Biology of the Cell, 15, 4829–4840.
Kondo, S., Murakami, T., Tatsumi, K., Ogata, M., Kanemoto, S., & Otori, K., et al. (2005). OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nature Cell Biology, 7, 186–194.
Kopito, R. R. (2000). Aggresomes, inclusion bodies and protein aggregation. Trends in Cell Biology, 10, 524–530.
Kouroku, Y., Fujita, E., Jimbo, A., Kikuchi, T., Yamagata, T., & Momoi, M. Y., et al. (2002). Polyglutamine aggregates stimulate ER stress signals and caspase-12 activation. Human Molecular Genetics, 11, 1505–1515.
Larsen, K. E., & Sulzer, D. (2002). Autophagy in neurons: A review. Histology and Histopathology, 17, 897–908.
Lee, A. H., Iwakoshi, N. N., Anderson, K. C., & Glimcher, L. H. (2003). Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 9946–9951.
Lee, S., & Tsai, F. T. (2005). Molecular chaperones in protein quality control. Journal of Biochemistry and Molecular Biology, 38, 259–265.
Leigh, P. N., Whitwell, H., Garofalo, O., Buller, J., Swash, M., & Martin, J. E., et al. (1991). Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis. Morphology, distribution, and specificity. Brain, 114(Pt 2), 775–788.
Levine, B., & Yuan, J. (2005). Autophagy in cell death: An innocent convict? Journal of Clinical Investigation, 115, 2679–2688.
Ma, J., & Lindquist, S. (2002). Conversion of PrP to a self-perpetuating PrPSc-like conformation in the cytosol. Science, 298, 1785–1788.
Ma, J., Wollmann, R., & Lindquist, S. (2002). Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science, 298, 1781–1785.
Mayer, M. P., & Bukau, B. (2005). Hsp70 chaperones: Cellular functions and molecular mechanism. Cellular and Molecular Life Sciences, 62, 670–684.
McConlogue, L., Castellano, F., deWit, C., Schenk, D., & Maltese, W. A. (1996). Differential effects of a Rab6 mutant on secretory versus amyloidogenic processing of Alzheimer’s beta-amyloid precursor protein. Journal of Biological Chemistry, 271, 1343–1348.
McNaught, K. S., Olanow, C. W. (2006). Proteasome inhibitor-induced model of Parkinson’s disease. Annals of Neurology, 60, 243–247.
McNaught, K. S., Perl, D. P., Brownell, A. L., & Olanow, C. W. (2004). Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson’s disease. Annals of Neurology, 56, 149–162.
Menendez-Benito, V., Verhoef, L. G., Masucci, M. G., & Dantuma, N. P. (2005). Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Human Molecular Genetics, 14, 2787–2799.
Meusser, B., Hirsch, C., Jarosch, E., & Sommer, T. (2005). ERAD: The long road to destruction. Nature Cell Biology, 7, 766–772.
Muratani, M., & Tansey, W. P. (2003). How the ubiquitin-proteasome system controls transcription. Nature Reviews. Molecular Cell Biology, 4, 192–201.
Nakagawa, T., Zhu, H., Morishima, N., Li, E., Xu, J., & Yankner, B. A., et al. (2000). Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature, 403, 98–103.
Ng, D. T., Spear, E. D., & Walter, P. (2000). The unfolded protein response regulates multiple aspects of secretory and membrane protein biogenesis and endoplasmic reticulum quality control. Journal of Cell Biology, 150, 77–88.
Nishitoh, H., Matsuzawa, A., Tobiume, K., Saegusa, K., Takeda, K., & Inoue, K., et al. (2002). ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes & Development, 16, 1345–1355.
Nixon, R. A. (2006). Autophagy in neurodegenerative disease: Friend, foe or turncoat? Trends in Neurosciences, 29, 528–535.
Nixon, R. A., Wegiel, J., Kumar, A., Yu, W. H., Peterhoff, C., & Cataldo, A., et al. (2005). Extensive involvement of autophagy in Alzheimer disease: An immuno-electron microscopy study. Journal of Neuropathology and Experimental Neurology, 64, 113–122.
Oddo, S., Billings, L., Kesslak, J. P., Cribbs, D. H., & LaFerla, F. M. (2004). Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron, 43, 321–332.
Ogata, M., Hino, S., Saito, A., Morikawa, K., Kondo, S., & Kanemoto, S., et al. (2006). Autophagy is activated for cell survival after endoplasmic reticulum stress. Molecular and Cellular Biology, 26, 9220–9231.
Olanow, C. W., Perl, D. P., DeMartino, G. N., & McNaught, K. S. (2004). Lewy-body formation is an aggresome-related process: A hypothesis. Lancet Neurology, 3, 496–503.
Orsi, A., Fioriti, L., Chiesa, R., & Sitia, R. (2006). Conditions of endoplasmic reticulum stress favor the accumulation of cytosolic prion protein. Journal of Biological Chemistry, 13, 30431–30438.
Piccini, A., Fassio, A., Pasqualetto, E., Vitali, A., Borghi, R., & Palmieri, D., et al. (2004). Fibroblasts from FAD-linked presenilin 1 mutations display a normal unfolded protein response but overproduce Abeta42 in response to tunicamycin. Neurobiology of Disease, 15, 380–386.
Pickart, C. M., & Cohen, R. E. (2004). Proteasomes and their kin: Proteases in the machine age. Nature Reviews. Molecular Cell Biology, 5, 177–187.
Reggiori, F., & Klionsky, D. J. (2005). Autophagosomes: Biogenesis from scratch? Current Opinion in Cell Biology, 17, 415–422.
Richly, H., Rape, M., Braun, S., Rumpf, S., Hoege, C., & Jentsch, S. (2005). A series of ubiquitin binding factors connects CDC48/p97 to substrate multiubiquitylation and proteasomal targeting. Cell, 120, 73–84.
Richter-Landsberg, C., & Goldbaum, O. (2003). Stress proteins in neural cells: Functional roles in health and disease. Cellular and Molecular Life Sciences, 60, 337–349.
Ryu, E. J., Harding, H. P., Angelastro, J. M., Vitolo, O. V., Ron, D., & Greene, L. A. (2002). Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. Journal of Neuroscience, 22, 10690–10698.
Scheper, W., & Hol, E. M. (2005). Protein quality control in Alzheimer’s disease: A fatal saviour. Current Drug Targets. CNS & Neurological Disorders, 4, 283–292.
Scheper, W., Hoozemans, J. J. M., Hoogenraad, C. C., Rozemuller, A. J. M., Eikelenboom, P., & Baas, F. (2007). Rab6 is increased in Alzheimer’s disease brain and correlates with endoplasmic reticulum stress. Neuropathology & Applied Neurobiology. (in press).
Schmitz, A., Schneider, A., Kummer, M. P., & Herzog, V. (2004). Endoplasmic reticulum-localized amyloid beta-peptide is degraded in the cytosol by two distinct degradation pathways. Traffic, 5, 89–101.
Schroder, M., & Kaufman, R. J. (2005). The mammalian unfolded protein response. Annual Review of Biochemistry, 74, 739–789.
Sikorska, B., Liberski, P. P., Giraud, P., Kopp, N., & Brown, P. (2004). Autophagy is a part of ultrastructural synaptic pathology in Creutzfeldt-Jakob disease: A brain biopsy study. International Journal of Biochemistry & Cell Biology, 36, 2563–2573.
Sittler, A., Lurz, R., Lueder, G., Priller, J., Lehrach, H., & Hayer-Hartl, M. K., et al. (2001). Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington’s disease. Human Molecular Genetics, 10, 1307–1315.
Soti, C., Nagy, E., Giricz, Z., Vigh, L., Csermely, P., & Ferdinandy, P. (2005). Heat shock proteins as emerging therapeutic targets. British Journal of Pharmacology, 146, 769–780.
Stege, G. J., Renkawek, K., Overkamp, P. S., Verschuure, P., van Rijk, A. F., & Reijnen-Aalbers, A., et al. (1999). The molecular chaperone alphaB-crystallin enhances amyloid beta neurotoxicity. Biochemical and Biophysical Research Communications, 262, 152–156.
Suen, K. C., Lin, K. F., Elyaman, W., So, K. F., Chang, R. C., & Hugon, J. (2003a). Reduction of calcium release from the endoplasmic reticulum could only provide partial neuroprotection against beta-amyloid peptide toxicity. Journal of Neurochemistry, 87, 1413–1426.
Suen, K. C., Yu, M. S., So, K. F., Chang, R. C., & Hugon, J. (2003b). Upstream signaling pathways leading to the activation of double-stranded RNA-dependent serine/threonine protein kinase in beta-amyloid peptide neurotoxicity. Journal of Biological Chemistry, 278, 49819–49827.
Takahashi, R., & Imai, Y. (2003). Pael receptor, endoplasmic reticulum stress, and Parkinson’s disease. Journal of Neurology, 250(Suppl 3), 25–29.
Tarabal, O., Caldero, J., Casas, C., Oppenheim, R. W., & Esquerda, J. E. (2005). Protein retention in the endoplasmic reticulum, blockade of programmed cell death and autophagy selectively occur in spinal cord motoneurons after glutamate receptor-mediated injury. Molecular and Cellular Neurosciences, 29, 283–298.
Thomas, M., Yu, Z., Dadgar, N., Varambally, S., Yu, J., & Chinnaiyan, A. M., et al. (2005). The unfolded protein response modulates toxicity of the expanded glutamine androgen receptor. Journal of Biological Chemistry, 280, 21264–21271.
Thrower, J. S., Hoffman, L., Rechsteiner, M., & Pickart, C. M. (2000). Recognition of the polyubiquitin proteolytic signal. EMBO Journal, 19, 94–102.
Tobisawa, S., Hozumi, Y., Arawaka, S., Koyama, S., Wada, M., & Nagai, M., et al. (2003). Mutant SOD1 linked to familial amyotrophic lateral sclerosis, but not wild-type SOD1, induces ER stress in COS7 cells and transgenic mice. Biochemical and Biophysical Research Communications, 303, 496–503.
Tyson, J. R., & Stirling, C. J. (2000). LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum. EMBO Journal, 19, 6440–6452.
Unterberger, U., Hoftberger, R., Gelpi, E., Flicker, H., Budka, H., & Voigtlander, T. (2006). Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. Journal of Neuropathology and Experimental Neurology, 65, 348–357.
Urushitani, M., Kurisu, J., Tateno, M., Hatakeyama, S., Nakayama, K., & Kato, S., et al. (2004). CHIP promotes proteasomal degradation of familial ALS-linked mutant SOD1 by ubiquitinating Hsp/Hsc70. Journal of Neurochemistry, 90, 231–244.
Urushitani, M., Kurisu, J., Tsukita, K., & Takahashi, R. (2002). Proteasomal inhibition by misfolded mutant superoxide dismutase 1 induces selective motor neuron death in familial amyotrophic lateral sclerosis. Journal of Neurochemistry, 83, 1030–1042.
van Leeuwen, F. W., de Kleijn, D. P., van den Hurk, H. H., Neubauer, A., Sonnemans, M. A., & Sluijs, J. A., et al. (1998). Frameshift mutants of beta amyloid precursor protein and ubiquitin-B in Alzheimer’s and Down patients. Science, 279, 242–247.
Varshavsky, A. (2005). Regulated protein degradation. Trends in Biochemical Sciences, 30, 283–286.
Vlug, A. S., Teuling, E., Haasdijk, E. D., French, P., Hoogenraad, C. C., & Jaarsma, D. (2005). ATF3 expression precedes death of spinal motoneurons in amyotrophic lateral sclerosis-SOD1 transgenic mice and correlates with c-Jun phosphorylation, CHOP expression, somato-dendritic ubiquitination and Golgi fragmentation. European Journal of Neuroscience, 22, 1881–1894.
Waelter, S., Boeddrich, A., Lurz, R., Scherzinger, E., Lueder, G., & Lehrach, H., et al. (2001). Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Molecular Biology of the Cell, 12, 1393–1407.
White, J., Johannes, L., Mallard, F., Girod, A., Grill, S., & Reinsch, S., et al. (1999). Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. Journal of Cell Biology, 147, 743–760.
Wiseman, R. L., & Balch, W. E. (2005). A new pharmacology-drugging stressed folding pathways. Trends in Molecular Medicine, 11, 347–350.
Wojcik, C., Schroeter, D., Wilk, S., Lamprecht, J., & Paweletz, N. (1996). Ubiquitin-mediated proteolysis centers in HeLa cells: Indication from studies of an inhibitor of the chymotrypsin-like activity of the proteasome. European Journal of Cell Biology, 71, 311–318.
Wootz, H., Hansson, I., Korhonen, L., Napankangas, U., & Lindholm, D. (2004). Caspase-12 cleavage and increased oxidative stress during motoneuron degeneration in transgenic mouse model of ALS. Biochemical and Biophysical Research Communications, 322, 281–286.
Yi, J. J., & Ehlers, M. D. (2005). Ubiquitin and protein turnover in synapse function. Neuron, 47, 629–632.
Yokota, T., Sugawara, K., Ito, K., Takahashi, R., Ariga, H., & Mizusawa, H. (2003). Down regulation of DJ-1 enhances cell death by oxidative stress, ER stress, and proteasome inhibition. Biochemical and Biophysical Research Communications, 312, 1342–1348.
Yoneda, T., Urano, F., & Ron, D. (2002). Transmission of proteotoxicity across cellular compartments. Genes & Development, 16, 1307–1313.
Yorimitsu, T., Nair, U., Yang, Z., & Klionsky, D. J. (2006). Endoplasmic reticulum stress triggers autophagy. Journal of Biological Chemistry, 281, 30299–30304.
Yu, M. S., Suen, K. C., Kwok, N. S., So, K. F., Hugon, J., & Chuen-Chung Chang, R. (2006). Beta-amyloid peptides induces neuronal apoptosis via a mechanism independent of unfolded protein responses. Apoptosis, 11, 687–700.
Yu, W. H., Cuervo, A. M., Kumar, A., Peterhoff, C. M., Schmidt, S. D., & Lee, J. H., et al. (2005). Macroautophagy-a novel {beta}-amyloid peptide-generating pathway activated in Alzheimer’s disease. Journal of Cell Biology, 171, 87–98.
Yu, W. H., Kumar, A., Peterhoff, C., Shapiro, K. L., Uchiyama, Y., & Lamb, B. T., et al. (2004). Autophagic vacuoles are enriched in amyloid precursor protein-secretase activities: Implications for beta-amyloid peptide over-production and localization in Alzheimer’s disease. International Journal of Biochemistry & Cell Biology, 36, 2531–2540.
Yu, Z., Luo, H., Fu, W., & Mattson, M. P. (1999). The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: Suppression of oxidative stress and stabilization of calcium homeostasis. Experimental Neurology, 155, 302–314.
Zhao, L., Longo-Guess, C., Harris, B. S., Lee, J. W., & Ackerman, S. L. (2005). Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nature Genetics, 37, 974–979.
Zinszner, H., Kuroda, M., Wang, X., Batchvarova, N., Lightfoot, R. T., & Remotti, H., et al. (1998). CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes & Development, 12, 982–995.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hol, E.M., Scheper, W. Protein Quality Control in Neurodegeneration: Walking the Tight Rope Between Health and Disease. J Mol Neurosci 34, 23–33 (2008). https://doi.org/10.1007/s12031-007-0013-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-007-0013-8