Abstract
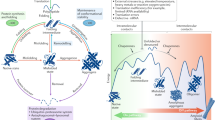
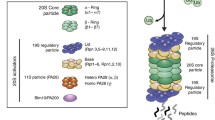
Cellular homeostasis is maintained by rapid and systematic cleansing of aberrant and aggregated proteins within cells. Neurodegenerative diseases (NDs) especially Parkinson’s and Alzheimer’s disease are known to be associated with multiple factors, most important being impaired clearance of aggregates, resulting in the accumulation of specific aggregated protein in the brain. Protein quality control (PQC) of proteostasis network comprises proteolytic machineries and chaperones along with their regulators to ensure precise operation and maintenance of proteostasis. Such regulatory factors coordinate among each other multiple functional aspects related to proteins, including their synthesis, folding, transport, and degradation. During aging due to inevitable endogenous and external stresses, sustaining a proteome balance is a challenging task. Such stresses decline the capacity of the proteostasis network compromising the proteome integrity, affecting the fundamental physiological processes including reproductive fitness of the organism. This review focuses on highlighting proteome-wide changes during aging and the strategies for proteostasis improvements. The possibility of augmenting the proteostasis network either via genetic or pharmacological interventions may be a promising strategy towards delaying age-associated pathological consequences due to proteome disbalance, thus promoting healthy aging and prolonged longevity.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Acosta, J. C., Banito, A., Wuestefeld, T., Georgilis, A., Morton, J. P., Athineos, D., Kang, T., Lasitschka, F., Andrulis, M., Pascual, G., Morris, K. J., Khan, S., Jin, H., Dharmalingam, G., Snijders, A. P., Carroll, T., Capper, D., Pritchard, C., Inman, G. J., Longerich, T., Sansom, O. J., Benitah, A., Zender, L., and Gil, J. (2014) Europe PMC Funders Group Europe PMC Funders Author Manuscripts A complex secretory program orchestrated by the inflammasome controls paracrine senescence.15, 978–990
Adam Bohnert K, Kenyon C (2017) A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage. Nature 551:629–633
Aguilera-Gomez A, Rabouille C (2017) Membrane-bound organelles versus membrane-less compartments and their control of anabolic pathways in Drosophila. Dev Biol 428:310–317
Alberti S, Hyman AA (2016) Are aberrant phase transitions a driver of cellular aging? BioEssays 38:959–968
Alfaro IE, Albornoz A, Molina A, Moreno J, Cordero K, Criollo A, Budini M (2019) Chaperone mediated autophagy in the crosstalk of neurodegenerative diseases and metabolic disorders. Front Endocrinol (Lausanne) 10:1–13
Anguiano M, Nowak RJ, Lansbury PT (2002) Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry 41:11338–11343
Arbor A (2006) Autophagy: is it cancer’s friend or foe? Science 80(312):1160–1161
Arndt V, Rogon C, Höhfeld J (2007) To be, or not to be - molecular chaperones in protein degradation. Cell Mol Life Sci 64:2525–2541
Badadani M (2012) Autophagy mechanism, regulation, functions, and disorders. ISRN Cell Biol 2012:1–11
Balch WE, Morimoto RI, Dillin A, Kelly JW (2008) Adapting proteostasis for disease intervention. Science 80(319):916–919
Balchin, D., Hayer-Hartl, M., and Hartl, F. U. (2016) In vivo aspects of protein folding and quality control. Science (80-. ). https://doi.org/10.1126/science.aac4354
Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM (2008) The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol 28:5747–5763
Baranczak A, Kelly JW (2016) A current pharmacologic agent versus the promise of next generation therapeutics to ameliorate protein misfolding and/or aggregation diseases. Curr Opin Chem Biol 32:10–21
Bergamini E, Cavallini G, Donati A, Gori Z (2004) The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int J Biochem Cell Biol 36:2392–2404
Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M (2010) Autophosphorylated CaMKIIα acts as a scaffold to recruit proteasomes to dendritic spines. Cell 140:567–578
Bogyo M, Gaczynska M, Ploegh HL (1997) Proteasome inhibitors and antigen presentation. Biopolymers 43:269–280
Brandt T, Mourier A, Tain LS, Partridge L, Larsson NG, Kühlbrandt W (2017) Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. Elife 6:1–19
Bronstein JM, Chou AP (2006) Peripheral proteasome and caspase activity in Parkinson disease and Alzheimer disease [1]. Neurology 67:182
Buchan JR, Kolaitis RM, Taylor JP, Parker R (2013) XEukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 153:1461
Cadonic C, Sabbir MG, Albensi BC (2016) Mechanisms of Mitochondrial dysfunction in Alzheimer’s disease. Mol Neurobiol 53:6078–6090
Carra S, Alberti S, Arrigo PA, Benesch JL, Benjamin IJ, Boelens W, Bartelt-Kirbach B, Brundel BJJM, Buchner J, Bukau B, Carver JA, Ecroyd H, Emanuelsson C, Finet S, Golenhofen N, Goloubinoff P, Gusev N, Haslbeck M, Hightower LE, Kampinga HH, Klevit RE, Liberek K, Mchaourab HS, McMenimen KA, Poletti A, Quinlan R, Strelkov SV, Toth ME, Vierling E, Tanguay RM (2017) The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones 22:601–611
Chang HW, Kwon S, Kim H, Lee K, Kim M, Moon T, Baek S (2002) Platelet-activating factor acetylhydrolase activity in cerebrospinal fluid of children with acute systemic or neurological illness. Ann Neurol 51:760–763
Chauhan R, Chen KF, Kent BA, Crowther DC (2017) Central and peripheral circadian clocks and their role in Alzheimer’s disease. DMM Dis Model Mech 10:1187–1199
Chong Y (1997) Effect of a carboxy-terminal fragment of the Alzheimer’s amyloid precursor protein on expression of proinflammatory cytokines in rat glial cells. Life Sci 61:2323–2333
Chee Yeun Chung,1* Vikram Khurana,1,2* Pavan K. Auluck,1,3 Daniel F. Tardiff,1 Joseph R. Mazzulli,2 Frank Soldner,1 Valeriya Baru,1,4 Yali Lou,1,4 Yelena Freyzon,1 Sukhee Cho,5 Alison E. Mungenast,5 Julien Muffat,1 Maisam Mitalipova,1 Michael D. Pluth,6 N, S. L. (2010) Identification and rescue of a-synuclein toxicity in Parkinson patient–derived neurons. 342, 983–987
Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD (2003) Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40:595–607
Cookson MR (2012) Parkinsonism due to mutations in PINK1, Parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harb Perspect Med 2:1–11
Cuervo AM (2004) Autophagy: in sickness and in health. Trends Cell Biol 14:70–77
Daniel MW, Kalfalah F, Florea AM, Sass S, Kruse F, Rieder V, Tigges J, Fritsche E, Krutmann J, Busch H, Meyer HE, Boege F, Theis F, Reifenberger G (2014) Proteome-wide analysisreveals an age-associated cellular phenotype. Aging 6:856–872
Demarest SJ, Martinez-Yamout M, Chung J, Chen H, Xu W, Jane Dyson H, Evans RM, Wright PE (2002) Mutual synergistic folding in recruitment of cbp/p300 by p160 nuclear receptor coactivators. Nature 415:549–553
Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, D’Amelio M, Nardacci R, Romagnoli A, Piacentini M, Cecconi F, Fimia GM (2010) The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol 191:155–168
Dice, J. F. UNIT 11 lysosomal pathways of protein degradation
Dick FD (2006) Parkinson’s disease and pesticide exposures. Br Med Bull 79–80:219–231
Dikic I (2017) Proteasomal and autophagic degradation systems. Annu Rev Biochem 86:193–224
Dunker AK, Silman I, Uversky VN, Sussman JL (2008) Function and structure of inherently disordered proteins. Curr Opin Struct Biol 18:756–764
Dunn WA (1990) Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J Cell Biol 110:1935–1945
Ehlers MD (2003) Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci 6:231–242
Esser C, Alberti S, Höhfeld J (2004) Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim Biophys Acta - Mol Cell Res 1695:171–188
Etlinger JD, Goldberg AL (1977) A soluble ATP dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A 74:54–58
Faragher, R. G. A., McArdle, A., Willows, A., and Ostler, E. L. (2017) Senescence in the aging process. F1000Research. 6, 1–9
Faust JR, Luskey KL, Chin DJ, Goldstein JL, Brown MS (1982) Regulation of synthesis and degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by low density lipoprotein and 25-hydroxycholesterol in UT-1 cells. Proc Natl Acad Sci U S A 79:5205–5209
Fearon RMP, Reiss D, Leve LD, Shaw DS, Scaramella LV, Ganiban JM, Neiderhiser JM (2015) Increased proteasome activity determines human embryonic stem cell identity. Dev Psychopathol 27:1251–1265
Feng Y, He D, Yao Z, Klionsky DJ (2014) The machinery of macroautophagy. Cell Res 24:24–41
Feng T, Tammineni P, Agrawal C, Jeong YY, Cai Q (2017) Autophagy-mediated regulation of BACE1 protein trafficking and degradation. J Biol Chem 292:1679–1690
Ganassi M, Mateju D, Bigi I, Mediani L, Poser I, Lee HO, Seguin SJ, Morelli FF, Vinet J, Leo G, Pansarasa O, Cereda C, Poletti A, Alberti S, Carra S (2016) A surveillance function of the HSPB8-BAG3-HSP70 chaperone complex ensures stress granule integrity and dynamism. Mol Cell 63:796–810
Gao W, Ding WX, Stolz DB, Yin XM (2008) Induction of macroautophagy by exogenously introduced calcium. Autophagy 4:754–761
García-Cerro S, Rueda N, Vidal V, Lantigua S, Martínez-Cué C (2017) Normalizing the gene dosage of Dyrk1A in a mouse model of Down syndrome rescues several Alzheimer’s disease phenotypes. Neurobiol Dis 106:76–88
F Gasset-rosa C Chillon-marinas A Goginashvili S Atwal JW Artates R Tabet VC Wheeler G Anne DW Cleveland C Lagier-tourenne L Jolla L Jolla MG Hospital MG Hospital S Burnham P Medical L Jolla 2018 HHS Public Access 94 48 57
Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12:119–131
Ghaemmaghami S et al (2003) Global analysis of protein expression in yeast. Nature 425:737–741
Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI (2006) Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311:1471–1474
-. Glover, D., Tajima, S., Yamamoto, A., Borgstrom, B., Brockman, L., Schotz, C., Guidoni, A., Caro, J. De, Crea, R., Acids, N., Sanger, F., Nicklen, S., Sarnbrook, J., and Harbor, C. S. (1986) Hiroshi mori,. 329, 0–3
Godin JD, Creppe C, Laguesse S, Nguyen L (2016) Emerging roles for the unfolded protein response in the developing nervous system. Trends Neurosci 39:394–404
Grice GL, Nathan JA (2016) The recognition of ubiquitinated proteins by the proteasome. Cell Mol Life Sci 73:3497–3506
Groll M, Bajorek M, Köhler A, Moroder L, Rubin DM, Huber R, Glickman MH, Finley D (2000) A gated channel into the proteasome core particle. Nat Struct Biol 7:1062–1067
Gutiérrez-Casado E, Khraiwesh H, López-Domínguez JA, Montero-Guisado J, López-Lluch G, Navas P, De Cabo R, Ramsey JJ, González-Reyes JA, Villalba JM (2019) The impact of aging, calorie restriction and dietary fat on autophagy markers and mitochondrial ultrastructure and dynamics in mouse skeletal muscle. J Gerontol. – Ser A Biol Sci Med Sci 74:760–769
Hara, K., Maruki, Y., Long, X., Yoshino, K. ichi, Oshiro, N., Hidayat, S., Tokunaga, C., Avruch, J., and Yonezawa, K. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 110, 177–189
Hasson SA, Kane LA, Yamano K, Huang CH, Sliter DA, Buehler E, Wang C, Heman-Ackah SM, Hessa T, Guha R, Martin SE, Youle RJ (2013) High-content genome-wide RNAi screens identify regulators of parkin upstream of mitophagy. Nature 504:291–295
Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15:233–249
Hipp MS, Kasturi P, Hartl FU (2019) The proteostasis network and its decline in ageing. Nat Rev Mol Cell Biol 20:421–435
Hoozemans JJM, Van Haastert ES, Nijholt DAT, Rozemuller AJM, Scheper W (2012) Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis 10:212–215
Jenkinson EM, Rehman AU, Walsh T, Clayton-Smith J, Lee K, Morell RJ, Drummond MC, Khan SN, Naeem MA, Rauf B, Billington N, Schultz JM, Urquhart JE, Lee MK, Berry A, Hanley NA, Mehta S, Cilliers D, Clayton PE, Kingston H, Smith MJ, Warner TT, Black GC, Trump D, Davis JRE, Ahmad W, Leal SM, Riazuddin S, King MC, Friedman TB, Newman WG (2013) Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am J Hum Genet 92:605–613
Jiao W, Li P, Zhang J, Zhang H, Chang Z (2005) Small heat-shock proteins function in the insoluble protein complex. Biochem Biophys Res Commun 335:227–231
Kedersha N, Ivanov P, Anderson P (2013) Stress granules and cell signaling: more than just a passing phase? Trends Biochem. Science 38:494–506
Kenyon CJ (2010) The genetics of ageing. Nature 464:504–512
Kenyon C (2011) The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos Trans R Soc B Biol Sci 366:9–16
Kim YE, Hosp F, Frottin F, Ge H, Mann M, Hayer-Hartl M, Hartl FU (2016) Soluble oligomers of polyQ-expanded huntingtin target a multiplicity of key cellular factors. Mol Cell 63:951–964
Kirstein-Miles J, Scior A, Deuerling E, Morimoto RI (2013) The nascent polypeptide-associated complex is a key regulator of proteostasis. EMBO J 32:1451–1468
Kisselev AF, Goldberg AL (2001) Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8:739–758
Kitada T, Pisani A, Karouani M, Haburcak M, Martella G, Tscherter A, Platania P, Wu B, Pothos EN, Shen J (2009) Impaired dopamine release and synaptic plasticity in the striatum of Parkin-/- mice. J Neurochem 110:613–621
Kitamura A, Inada N, Kubota H, Matsumoto G, Kinjo M, Morimoto RI, Nagata K (2014) Dysregulation of the proteasome increases the toxicity of ALS-linked mutant SOD1. Genes Cells 19:209–224
Klaips CL, Jayaraj GG, Hartl FU (2018) Pathways of cellular proteostasis in aging and disease. J Cell Biol 217:51–63
Kostova KK, Hickey KL, Osuna BA, Hussmann JA, Frost A, Weinberg DE, Weissman JS (2017) CAT-tailing as a fail-safe mechanism for efficient degradation of stalled nascent polypeptides. Science 80(357):414–417
Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38:518–520
Kulak NA, Geyer PE, Mann M (2017) Loss-less nano-fractionator for high sensitivity, high coverage proteomics. Mol Cell Proteomics 16:694–705
Kumar KR, Weissbach A, Heldmann M, Kasten M, Tunc S, Sue CM, Svetel M, Kostić VS, Segura-Aguilar J, Ramirez A, Simon DK, Vieregge P, Münte TF, Hagenah J, Klein C, Lohmann K (2012) Frequency of the D620N mutation in VPS35 in Parkinson disease. Arch Neurol 69:1360–1364
Kundra R, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M (2017) Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. Proc Natl Acad Sci USA 114:E5703–E5711
Kunzt JB, Schwarz H, Mayer A (2004) Determination of four sequential Stages during microautophagy in vitro. J Biol Chem 279:9987–9996
Kuzuhara S, Mori H, Izumiyama N, Yoshimura M, Ihara Y (1988) Lewy bodies are ubiquitinated. Acta Neuropathol 75:345–353
Labbadia J, Morimoto RI (2015) Repression of the heat shock response is a programmed event at the onset of reproduction. Mol Cell 59:639–650
Labbadia J, Morimoto RI (2015) The biology of proteostasis in aging and disease. Annu Rev Biochem 84:435–464
Laguesse S, Creppe C, Nedialkova DD, Prévot PP, Borgs L, Huysseune S, Franco B, Duysens G, Krusy N, Lee G, Thelen N, Thiry M, Close P, Chariot A, Malgrange B, Leidel SA, Godin JD, Nguyen L (2015) A dynamic unfolded protein response contributes to the control of cortical neurogenesis. Dev Cell 35:553–567
Lamark, T., and Johansen, T. (2012) Aggrephagy: selective disposal of protein aggregates by macroautophagy. Int. J. Cell Biol. https://doi.org/10.1155/2012/736905
Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418:291
Lechler MC, Crawford ED, Groh N, Widmaier K, Jung R, Kirstein J, Trinidad JC, Burlingame AL, David DC (2017) Reduced insulin/IGF-1 signaling restores the dynamic properties of key stress granule proteins during aging. Cell Rep 18:454–467
Lee C, Kim H, Bardwell JCA (2018) Electrostatic interactions are important for chaperone–client interaction in vivo. Microbiol (United Kingdom) 164:992–997
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, Widner H, Revesz T, Lindvall O, Brundin P (2008) Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med 14:501–503
Li WW, Li J, Bao JK (2012) Microautophagy: lesser-known self-eating. Cell Mol Life Sci 69:1125–1136
Liang Y (2019) Emerging concepts and functions of autophagy as a regulator of synaptic components and plasticity. Cells 8:34
Liang YT, Sigrist S (2018) Autophagy and proteostasis in the control of synapse aging and disease. Curr Opin Neurobiol 48:113–121
Liang V, Ullrich M, Lam H, Chew YL, Banister S, Song X, Zaw T, Kassiou M, Götz J, Nicholas HR (2014) Altered proteostasis in aging and heat shock response in C. elegans revealed by analysis of the global and de novo synthesized proteome. Cell Mol Life Sci 71:3339–3361
Liu Q, D’Silva P, Walter W, Marszalek J, Craig EA (2003) Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science 80(300):139–141
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194
Lu K, den Brave F, Jentsch S (2017) Pathway choice between proteasomal and autophagic degradation. Autophagy 13:1799–1800
Lu K, Den Brave F, Jentsch S (2017) Receptor oligomerization guides pathway choice between proteasomal and autophagic degradation. Nat Cell Biol 19:732–739
Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors PA, Rautakorpi I, Peltonen PL, Majamaa PK, Somer H, Suomalainen A (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase γ mutations: clinical and molecular genetic study. Lancet 364:875–882
Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI (2007) Macromolecule biosynthesis: a key function of sleep. Physiol Genomics 31:441–457
Manderville RA, Wetmore SD (2017) Mutagenicity of ochratoxin A: role for a carbon-linked C8-deoxyguanosine adduct? J Agric Food Chem 65:7097–7105
Martínez-Cué C, Rueda N (2020) Cellular senescence in neurodegenerative diseases. Front Cell Neurosci. https://doi.org/10.3389/fncel.2020.00016
Matai L, Sarkar GC, Chamoli M, Malik Y, Kumar SS, Rautela U, Jana NR, Chakraborty K, Mukhopadhyay A (2019) Dietary restriction improves proteostasis and increases life span through endoplasmic reticulum hormesis. Proc Natl Acad Sci USA 116:17383–17392
Mateju D, Franzmann TM, Patel A, Kopach A, Boczek EE, Maharana S, Lee HO, Carra S, Hyman AA, Alberti S (2017) An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J 36:1669–1687
Mimura N, Yuasa S, Soma M, Jin H, Kimura K, Goto S, Koseki H, Aoe T (2008) Altered quality control in the endoplasmic reticulum causes cortical dysplasia in knock-in mice expressing a mutant BiP. Mol Cell Biol 28:293–301
Minard AY, Wong MKL, Chaudhuri R, Tan SX, Humphrey SJ, Parker BL, Yang JY, Laybutt DR, Cooney GJ, Coster ACF, Stöckli J, James DE (2016) Hyperactivation of the insulin signaling pathway improves intracellular proteostasis by coordinately up-regulating the proteostatic machinery in adipocytes. J Biol Chem 291:25629–25640
Moehle EA, Shen K, Dillin A (2019) Mitochondrial proteostasis in the context of cellular and organismal health and aging. J Biol Chem 294:5396–5407
Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, Haynes CM, Chen D (2015) A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science 80(347):1374–1377
Moll L, Roitenberg N, Bejerano-Sagie M, Boocholez H, Marques FC, Volovik Y, Elami T, Siddiqui AA, Grushko D, Biram A, Lampert B, Achache H, Ravid T, Tzur YB, Cohen E (2018) The insulin/IGF signaling cascade modulates SUMOylation to regulate aging and proteostasis in caenorhabditis elegans. Elife 7:1–29
Morrow G, Tanguay RM (2015) Drosophila melanogaster Hsp22: a mitochondrial small heat shock protein influencing the aging process. Front Genet 6:1–7
Moujaber O, Mahboubi H, Kodiha M, Bouttier M, Bednarz K, Bakshi R, White J, Larose L, Colmegna I, Stochaj U (2017) Dissecting the molecular mechanisms that impair stress granule formation in aging cells. Biochim Biophys Acta - Mol Cell Res 1864:475–486
Nargund A, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM (2012) Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 80(337):587–590
Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM (2015) Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPRmt. Mol Cell 58:123–133
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, MacKenzie IRA (2009) A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132:2922–2931
Nikoletopoulou V, Sidiropoulou K, Kallergi E, Dalezios Y, Tavernarakis N (2017) Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab 26:230-242.e5
Opoku-Nsiah KA, Gestwicki JE (2018) Aim for the core: suitability of the ubiquitin-independent 20S proteasome as a drug target in neurodegeneration. Transl Res 198:48–57
Ori A, Toyama BH, Harris MS, Bock T, Iskar M, Bork P, Ingolia NT, Hetzer MW, Beck M (2015) Integrated transcriptome and proteome analyses reveal organ-specific proteome deterioration in old rats. Cell Syst 1:224–237
Ounallah-Saad H, Sharma V, Edry E, Rosenblum K (2014) Genetic or pharmacological reduction of PERK enhances cortical-dependent taste learning. J Neurosci 34:14624–14632
Paxman R, Plate L, Blackwood EA, Glembotski C, Powers ET, Wiseman RL, Kelly JW (2018) Pharmacologic ATF6 activating compounds are metabolically activated to selectively modify endoplasmic reticulum proteins. Elife 7:1–23
Pellegrino MW, Haynes CM (2015) Mitophagy and the mitochondrial unfolded protein response in neurodegeneration and bacterial infection. BMC Biol 13:1–9
Abhisek Mukherjee1, Diego Morales-Scheihing1, 2, Peter C. Butler3, and C. S. (2015) Type 2 diabetes as a misfolding disease. 155 3 12
Picotti P et al (2013) A complete mass-spectrometric map of the yeast proteome applied to quantitative trait analysis. Nature 494:266–270
Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78:959–991
Protter DSW, Parker R (2016) Principles and properties of stress granules. Trends Cell Biol 26:668–679
Rallis A, Navarro JA, Rass M, Hu A, Birman S, Schneuwly S, Thérond PP (2020) Hedgehog signaling modulates glial proteostasis and lifespan. Cell Rep 30:2627-2643.e5
Ramachandran KV, Margolis SS (2017) A mammalian nervous-system-specific plasma membrane proteasome complex that modulates neuronal function. Nat Struct Mol Biol 24:419–430
Ramachandran KV, Fu JM, Schaffer TB, Na CH, Delannoy M, Margolis SS (2018) Activity-dependent degradation of the nascentome by the neuronal membrane proteasome. Mol Cell 71:169-177.e6
Rangaraju S, Verrier JD, Madorsky I, Nicks J, Dunn WA, Notterpek L (2010) Rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice. J Neurosci 30:11388–11397
Ravikumar B, Futter M, Jahreiss L, Korolchuk VI, Lichtenberg M, Luo S, Massey DCO, Menzies FM, Narayanan U, Renna M, Jimenez-Sanchez M, Sarkar S, Underwood B, Winslow A, Rubinsztein DC (2009) Mammalian macroautophagy at a glance. J Cell Sci 122:1707–1711
Reis-Rodrigues P, Czerwieniec G, Peters TW, Evani US, Alavez S, Gaman EA, Vantipalli M, Mooney SD, Gibson BW, Lithgow GJ, Hughes RE (2012) Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell 11:120–127
Riek R, Eisenberg DS (2016) The activities of amyloids from a structural perspective. Nature 539:227–235
Ross Buchan J (2014) MRNP granules assembly, function, and connections with disease. RNA Biol 11:1019–1030
Rousseau A, Bertolotti A (2016) An evolutionarily conserved pathway controls proteasome homeostasis. Nature 536:184–189
Rubinsztein DC, Mariño G, Kroemer G (2011) Autophagy and aging. Cell 146:682–695
Saez I, Vilchez D (2014) The mechanistic links between proteasome activity, aging and agerelated diseases. Curr Genomics 15:38–51
Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L (2011) Microautophagy of cytosolic proteins by late endosomes. Dev Cell 20:131–139
Sauer RT, Baker TA (2011) AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem 80:587–612
Schmidt M, Finley D (2014) Regulation of proteasome activity in health and disease. Biochim Biophys Acta - Mol Cell Res 1843:13–25
Schubert U, Antón LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR (2000) Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404:770–774
Seaman MNJ (2004) Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol 165:111–122
Şentürk M, Lin G, Zuo Z, Mao D, Watson E, Mikos AG, Bellen HJ (2019) Ubiquilins regulate autophagic flux through mTOR signalling and lysosomal acidification. Nat Cell Biol 21:384–396
Shemesh N, Ben-Zvi A (2018) No excess baggage: new life starts with a clean slate. Mol Cell 69:163–164
Shpilka T, Haynes CM (2018) The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol 19:109–120
Song J, Herrmann JM, Becker T (2021) Quality control of the mitochondrial proteome. Nat Rev Mol Cell Biol 22:54–70
Stickgold R, Walker MP (2007) Sleep-dependent memory consolidation and reconsolidation. Sleep Med 8:331–343
Tabara K, Iwata Y, Koizumi N (2018) The unfolded protein response. Methods Mol Biol 1691:223–230
Taylor RC, Dillin A (2011) Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol 3:1–17
Terlecky SR, Chiang HL, Olson TS, Dice JF (1992) Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J Biol Chem 267:9202–9209
RE Thomas LA Andrews JL Burman WY Lin LJ Pallanck 2014 PINK1-Parkin pathway activity is regulated by degradation of PINK1 in the mitochondrial matrix PLoS Genet https://doi.org/10.1371/journal.pgen.1004279
Thrower JS, Hoffman L, Rechsteiner M, Pickart CM (2000) Recognition of the polyubiquitin proteolytic signal. EMBO J 19:94–102
Tononi G, Cirelli C (2003) Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull 62:143–150
Tsaytler P, Harding HP, Ron D, Bertolotti A (2011) Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 80(332):91–94
Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C (2013) When ER stress reaches a dead end. Biochim Biophys Acta - Mol Cell Res 1833:3507–3517
Urra H, Henriquez DR, Cánovas J, Villarroel-Campos D, Carreras-Sureda A, Pulgar E, Molina E, Hazari YM, Limia CM, Alvarez-Rojas S, Figueroa R, Vidal RL, Rodriguez DA, Rivera CA, Court FA, Couve A, Qi L, Chevet E, Akai R, Iwawaki T, Concha ML, Glavic Á, Gonzalez-Billault C, Hetz C (2018) IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat Cell Biol 20:942–953
Van Deursen JM (2014) The role of senescent cells in ageing. Nature 509:439–446
Velarde MC, Menon R (2016) Positive and negative effects of cellular senescence during female reproductive aging and pregnancy. J Endocrinol 230:R59–R76
Veljanovski V, Batoko H (2014) Selective autophagy of non-ubiquitylated targets in plants: looking for cognate receptor/adaptor proteins. Front Plant Sci 5:1–6
Ventura MT, Casciaro M, Gangemi S, Buquicchio R (2017) Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy 15:1–8
Verma R, Deshaies RJ (2000) A proteasome howdunit: the case of the missing signal. Cell 101:341–344
Von Zglinicki T, Pilger R, Sitte N (2000) Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Radic Biol Med 28:64–74
Voronina, E., Seydoux, G., Sassone-Corsi, P., and Nagamori, I. (2011) Cold Spring Harb Perspect Biol. RNA granules in germ cells subject collections
Walther DM, Mann M (2011) Accurate quantification of more than 4000 mouse tissue proteins reveals minimal proteome changes during aging. Mol Cell Proteomics 10:1–7
Williams KW, Liu T, Kong X, Fukuda M, Deng Y, Berglund ED, Deng Z, Gao Y, Liu T, Sohn JW, Jia L, Fujikawa T, Kohno D, Scott MM, Lee S, Lee CE, Sun K, Chang Y, Scherer PE, Elmquist JK (2014) Xbp1s in pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab 20:471–482
Winckler B, Faundez V, Maday S, Cai Q, Almeida CG, Zhang H (2018) The endolysosomal system and proteostasis: from development to degeneration. J Neurosci 38:9364–9374
Wrobel L, Topf U, Bragoszewski P, Wiese S, Sztolsztener ME, Oeljeklaus S, Varabyova A, Lirski M, Chroscicki P, Mroczek S, Januszewicz E, Dziembowski A, Koblowska M, Warscheid B, Chacinska A (2015) Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature 524:485–488
Yamamoto, A., and Lucas, J. (2000) Yamamoto et al. - 2000 - Cell . 101, 57–66
Young, J. C., Hoogenraad, N. J., and Hartl, F. U. (2005) Contents, Ed. Board + Forthc. articles. Trends Biochem. Sci. 30, i
Zhang K, Kaufman RJ (2004) Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem 279:25935–25938
Zhang R, Asai M, Mahoney CE, Joachim M, Shen Y, Gunner G, Majzoub JA (2016) Age- and hypertension-associated protein aggregates in mouse heart have similar proteomic profiles. Hypertension 22:733–744
Zheng X, Krakowiak J, Patel N, Beyzavi A, Ezike J, Khalil AS, Pincus D (2016) Dynamic control of HSF1 during heat shock by a chaperone switch and phosphorylation. Elife 5:1–26
Zhu D, Wu X, Zhou J, Li X, Huang X, Li J, Wu J, Bian Q, Wang Y, Tian Y (2020) NuRD mediates mitochondrial stress-induced longevity via chromatin remodeling in response to acetyl-CoA level. Sci Adv 6:1–12
Acknowledgements
We are thankful to CSIR-CDRI for providing the infrastructure and access to the journals for writing this review.
Funding
AS is a Senior Research Fellow (CSIR) vide reference no EMR/No./31/004(1273)2014-EMR-I. AN acknowledges the funding received from CSIR-CDRI vide project MLP0020 (Neuroscience and Ageing Biology).
Author information
Authors and Affiliations
Contributions
AS conducted the research, analyzed data, and wrote the manuscript; AN conceived the study, provided infrastructure, analyzed the data, and edited the manuscript. All authors read the manuscript and agreed its content before the submission.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkar, A., Nazir, A. Carrying Excess Baggage Can Slowdown Life: Protein Clearance Machineries That Go Awry During Aging and the Relevance of Maintaining Them. Mol Neurobiol 59, 821–840 (2022). https://doi.org/10.1007/s12035-021-02640-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02640-2