Abstract
Purpose
Non-operative management of rectal cancer is a feasible and appealing treatment option for patients who develop a complete response after neoadjuvant therapy. However, identifying patients who are complete responders is often a challenge. This review aims to present and discuss current evidence and recommendations regarding the assessment of treatment response in rectal cancer.
Methods
A review of the current literature on rectal cancer restaging was performed. Studies included in this review explored the optimal interval between the end of neoadjuvant therapy and restaging, as well as modalities of assessment and their diagnostic performance.
Results
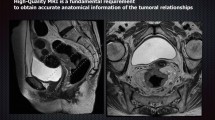
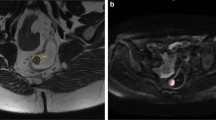
The current standard for restaging rectal cancer is a multimodal assessment with the digital rectal examination, endoscopy, and T2-weighted MRI with diffusion-weighted imaging. Other diagnostic procedures under investigation are PET/MRI, radiomics, confocal laser endomicroscopy, artificial intelligence-assisted endoscopy, cell-free DNA, and prediction models incorporating one or more of the above-mentioned exams.
Conclusion
Non-operative management of rectal cancer requires a multidisciplinary approach. Understanding of the robustness and limitations of each exam is critical to inform patient selection for that treatment strategy.



Similar content being viewed by others
References
Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–8. https://doi.org/10.1097/01.sla.0000141194.27992.32.
Benson AB, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(10):1139–67. https://doi-org.laneproxy.stanford.edu/10.6004/jnccn.2022.0051
van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. The Lancet. 2018;391(10139):2537–45. https://doi.org/10.1016/S0140-6736(18)31078-X.
Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174–83. https://doi.org/10.1016/S1470-2045(15)00467-2.
Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2017;2(7):501–13. https://doi.org/10.1016/S2468-1253(17)30074-2.
Bahadoer RR, Peeters KCMJ, Beets GL, et al. Watch and wait after a clinical complete response in rectal cancer patients younger than 50 years. Br J Surg. 2021;109(1):114–20. https://doi.org/10.1093/bjs/znab372.
Cui CL, Luo WY, Cosman BC, et al. Cost effectiveness of watch and wait versus resection in rectal cancer patients with complete clinical response to neoadjuvant chemoradiation. Ann Surg Oncol. 2022;29(3):1894–907. https://doi.org/10.1245/s10434-021-10576-z.
Kasi A, Abbasi S, Handa S, et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12): e2030097. https://doi.org/10.1001/jamanetworkopen.2020.30097.
Kong JC, Soucisse M, Michael M, et al. Total Neoadjuvant therapy in locally advanced rectal cancer: a systematic review and metaanalysis of oncological and operative outcomes. Ann Surg Oncol. 2021;28(12):7476–86. https://doi.org/10.1245/s10434-021-09837-8.
Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440–8. https://doi.org/10.1097/SLA.0000000000003471.
Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. https://doi.org/10.1016/S1470-2045(20)30555-6.
Garcia-Aguilar J, Patil S, Gollub MJ, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. JCO. 2022;40(23):2546–56. https://doi.org/10.1200/JCO.22.00032.
Fokas E, Allgäuer M, Polat B, et al. Randomized phase II Trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. JCO. 2019;37(34):3212–22. https://doi.org/10.1200/JCO.19.00308.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26(8):1722–8. https://doi.org/10.1093/annonc/mdv223.
Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6): e180071. https://doi.org/10.1001/jamaoncol.2018.0071.
Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF, et al. Pathologic complete response in rectal cancer: can we detect it? Lessons learned from a proposed randomized trial of watch-and-wait treatment of rectal cancer. Dis Colon Rectum. 2016;59(4):255–63. https://doi.org/10.1097/DCR.0000000000000558.
Yu M, Wang DC, Li S, Huang LY, Wei J. Does a long interval between neoadjuvant chemoradiotherapy and surgery benefit the clinical outcomes of locally advanced rectal cancer? A systematic review and meta analyses. Int J Colorectal Dis. 2022;37(4):855–68. https://doi.org/10.1007/s00384-022-04122-w.
Gambacorta MA, Masciocchi C, Chiloiro G, et al. Timing to achieve the highest rate of pCR after preoperative radiochemotherapy in rectal cancer: a pooled analysis of 3085 patients from 7 randomized trials. Radiother Oncol. 2021;154:154–60. https://doi.org/10.1016/j.radonc.2020.09.026.
Hupkens BJP, Maas M, Martens MH, et al. Organ preservation in rectal cancer after chemoradiation: should we extend the observation period in patients with a clinical near-complete response? Ann Surg Oncol. 2018;25(1):197–203. https://doi.org/10.1245/s10434-017-6213-8.
Habr-Gama A, São Julião GP, Fernandez LM, et al. Achieving a complete clinical response after neoadjuvant chemoradiation that does not require surgical resection: it may take longer than you think! Dis Colon Rectum. 2019;62(7):802–8. https://doi.org/10.1097/DCR.0000000000001338.
Suzuki C, Halperin SK, Nilsson PJ, Martling A, Holm T. Initial magnetic resonance imaging tumour regression grade (mrTRG) as response evaluation after neoadjuvant treatment predicts sustained complete response in patients with rectal cancer. Eur J Surg Oncol. 2022;48(7):1643–9. https://doi.org/10.1016/j.ejso.2022.02.012.
Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete Clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692–8. https://doi.org/10.1007/DCR.0b013e3181f42b89.
Smith FM, Wiland H, Mace A, Pai RK, Kalady MF. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2014;57(3):311–5. https://doi.org/10.1097/DCR.0b013e3182a84eba.
Liu S, Zhong G xi, Zhou W xun, et al. Can endorectal ultrasound, MRI, and mucosa integrity accurately predict the complete response for mid-low rectal cancer after preoperative chemoradiation? A prospective observational study from a single medical center: Dis Colon Rectum. Published online June 2018:1. https://doi.org/10.1097/DCR.0000000000001135.
van der Sande ME, Maas M, Melenhorst J, Breukink SO, van Leerdam ME, Beets GL. Predictive value of endoscopic features for a complete response after chemoradiotherapy for rectal cancer. Ann Surg. 2021;274(6):e541–7. https://doi.org/10.1097/SLA.0000000000003718.
Duldulao MP, Lee W, Streja L, et al. Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum. 2013;56(2):142–9. https://doi.org/10.1097/DCR.0b013e31827541e2.
van der Sande ME, Beets GL, Hupkens BJP, et al. Response assessment after (chemo) radiotherapy for rectal cancer: why are we missing complete responses with MRI and endoscopy? Eur J Surg Oncol. 2019;45(6):1011–7. https://doi.org/10.1016/j.ejso.2018.11.019.
Safatle-Ribeiro AV, Marques CFS, Pires C, et al. Diagnosis of clinical complete response by probe-based confocal laser endomicroscopy (pCLE) after chemoradiation for advanced rectal cancer. J Gastrointest Surg. 2021;25(2):357–68. https://doi.org/10.1007/s11605-020-04878-y.
Haak HE, Gao X, Maas M, et al. The use of deep learning on endoscopic images to assess the response of rectal cancer after chemoradiation. Surg Endosc. 2022;36(5):3592–600. https://doi.org/10.1007/s00464-021-08685-7.
Beets-Tan RGH, Lambregts DMJ, Maas M, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–75. https://doi.org/10.1007/s00330-017-5026-2.
Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging–detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. JCO. 2011;29(28):3753–60. https://doi.org/10.1200/JCO.2011.34.9068.
Siddiqui MRS, Gormly KL, Bhoday J, et al. Interobserver agreement of radiologists assessing the response of rectal cancers to preoperative chemoradiation using the MRI tumour regression grading (mrTRG). Clin Radiol. 2016;71(9):854–62. https://doi.org/10.1016/j.crad.2016.05.005.
Memon S, Lynch AC, Bressel M, Wise AG, Heriot AG. Systematic review and meta-analysis of the accuracy of MRI and endorectal ultrasound in the restaging and response assessment of rectal cancer following neoadjuvant therapy. Colorectal Dis. 2015;17(9):748–61. https://doi.org/10.1111/codi.12976.
Zhao RS, Wang H, Zhou ZY, Zhou Q, Mulholland MW. Restaging of locally advanced rectal cancer with magnetic resonance imaging and endoluminal ultrasound after preoperative chemoradiotherapy: a systemic review and meta-analysis. Dis Colon Rectum. 2014;57(3):388–95. https://doi.org/10.1097/DCR.0000000000000022.
Sclafani F, Brown G, Cunningham D, et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer. 2017;117(10):1478–85. https://doi.org/10.1038/bjc.2017.320.
Park SH, Cho SH, Choi SH, et al. MRI Assessment of Complete response to preoperative chemoradiation therapy for rectal cancer: 2020 guide for practice from the Korean society of abdominal radiology. Korean J Radiol. 2020;21(7):812. https://doi.org/10.3348/kjr.2020.0483.
Lambregts DMJ, Delli Pizzi A, Lahaye MJ, et al. A pattern-based approach combining tumor morphology on mri with distinct signal patterns on diffusion-weighted imaging to assess response of rectal tumors after chemoradiotherapy. Dis Colon Rectum. 2018;61(3):328–37. https://doi.org/10.1097/DCR.0000000000000915.
Kim SH, Lee JM, Hong SH, et al. Locally Advanced rectal cancer: added value of diffusion-weighted mr imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253(1):116–25. https://doi.org/10.1148/radiol.2532090027.
Sassen S, de Booij M, Sosef M, et al. Locally advanced rectal cancer: is diffusion weighted MRI helpful for the identification of complete responders (ypT0N0) after neoadjuvant chemoradiation therapy? Eur Radiol. 2013;23(12):3440–9. https://doi.org/10.1007/s00330-013-2956-1.
van der Paardt MP, Zagers MB, Beets-Tan RGH, Stoker J, Bipat S. Patients Who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic mr imaging: a systematic review and meta-analysis. Radiology. 2013;269(1):101–12. https://doi.org/10.1148/radiol.13122833.
Foti PV, Privitera G, Piana S, et al. Locally advanced rectal cancer: qualitative and quantitative evaluation of diffusion-weighted MR imaging in the response assessment after neoadjuvant chemo-radiotherapy. European Journal of Radiology Open. 2016;3:145–52. https://doi.org/10.1016/j.ejro.2016.06.003.
Schurink NW, Lambregts DMJ, Beets-Tan RGH. Diffusion-weighted imaging in rectal cancer: current applications and future perspectives. BJR. 2019;92(1096):20180655. https://doi.org/10.1259/bjr.20180655.
Crimì F, Spolverato G, Lacognata C, et al. 18F-FDG PET/MRI for Rectal cancer TNM Restaging after preoperative chemoradiotherapy: initial experience. Dis Colon Rectum. 2020;63(3):310–8. https://doi.org/10.1097/DCR.0000000000001568.
Lee SJ, Seo HJ, Kang KW, et al. Clinical performance of whole-body 18F-FDG PET/Dixon-VIBE, T1-weighted, and T2-weighted MRI protocol in colorectal cancer. Clin Nucl Med. 2015;40(8):e392–8. https://doi.org/10.1097/RLU.0000000000000812.
Brendle C, Schwenzer NF, Rempp H, et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur J Nucl Med Mol Imaging. 2016;43(1):123–32. https://doi.org/10.1007/s00259-015-3137-z.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–77. https://doi.org/10.1148/radiol.2015151169.
Feng L, Liu Z, Li C, et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a multicentre observational study. Lancet Digit Health. 2022;4(1):e8–17. https://doi.org/10.1016/S2589-7500(21)00215-6.
Song M, Li S, Wang H, et al. MRI radiomics independent of clinical baseline characteristics and neoadjuvant treatment modalities predicts response to neoadjuvant therapy in rectal cancer. Br J Cancer. 2022;127(2):249–57. https://doi.org/10.1038/s41416-022-01786-7.
Miranda J, Tan GXV, Fernandes MC, et al. Rectal MRI radiomics for predicting pathological complete response: where we are. Clin Imaging. 2022;82:141–9. https://doi.org/10.1016/j.clinimag.2021.10.005.
Horvat N, Bates DDB, Petkovska I. Novel imaging techniques of rectal cancer: what do radiomics and radiogenomics have to offer? A literature review. Abdom Radiol. 2019;44(11):3764–74. https://doi.org/10.1007/s00261-019-02042-y.
Nardone V, Reginelli A, Guida C, et al. Delta-radiomics increases multicentre reproducibility: a phantom study. Med Oncol. 2020;37(5):38. https://doi.org/10.1007/s12032-020-01359-9.
Wan L, Peng W, Zou S, et al. MRI-based delta-radiomics are predictive of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Acad Radiol. 2021;28:S95–104. https://doi.org/10.1016/j.acra.2020.10.026.
Shayesteh S, Nazari M, Salahshour A, et al. Treatment response prediction using MRI-based pre-, post-, and delta-radiomic features and machine learning algorithms in colorectal cancer. Med Phys. 2021;48(7):3691–701. https://doi.org/10.1002/mp.14896.
Cusumano D, Boldrini L, Yadav P, et al. Delta radiomics for rectal cancer response prediction using low field magnetic resonance guided radiotherapy: an external validation. Physica Med. 2021;84:186–91. https://doi.org/10.1016/j.ejmp.2021.03.038.
Bulens P, Couwenberg A, Intven M, et al. Predicting the tumor response to chemoradiotherapy for rectal cancer: model development and external validation using MRI radiomics. Radiother Oncol. 2020;142:246–52. https://doi.org/10.1016/j.radonc.2019.07.033.
Shahzadi I, Zwanenburg A, Lattermann A, et al. Analysis of MRI and CT-based radiomics features for personalized treatment in locally advanced rectal cancer and external validation of published radiomics models. Sci Rep. 2022;12(1):10192. https://doi.org/10.1038/s41598-022-13967-8.
Maas M, Lambregts DMJ, Nelemans PJ, et al. Assessment of Clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol. 2015;22(12):3873–80. https://doi.org/10.1245/s10434-015-4687-9.
on behalf of the Rectal Cancer Consortium, Smith JJ, Chow OS, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15(1):767. https://doi.org/10.1186/s12885-015-1632-z.
Wallin U, Rothenberger D, Lowry A, Luepker R, Mellgren A. CEA – A predictor for pathologic complete response after neoadjuvant therapy for rectal cancer. Dis Colon Rectum. 2013;56(7):859–68. https://doi.org/10.1097/DCR.0b013e31828e5a72.
Huh JW, Kim HR, Kim YJ. Clinical Prediction of pathological complete response after preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum. 2013;56(6):698–703. https://doi.org/10.1097/DCR.0b013e3182837e5b.
Kleiman A, Al-Khamis A, Farsi A, et al. Normalization of CEA levels post-neoadjuvant therapy is a strong predictor of pathologic complete response in rectal cancer. J Gastrointest Surg. 2015;19(6):1106–12. https://doi.org/10.1007/s11605-015-2814-3.
Perez RO, São Julião GP, Habr-Gama A, et al. The role of carcinoembriogenic antigen in predicting response and survival to neoadjuvant chemoradiotherapy for distal rectal cancer. Dis Colon Rectum. 2009;52(6):1137–43. https://doi.org/10.1007/DCR.0b013e31819ef76b.
Li A, He K, Guo D, et al. Pretreatment blood biomarkers predict pathologic responses to neo-CRT in patients with locally advanced rectal cancer. Future Oncol. 2019;15(28):3233–42. https://doi.org/10.2217/fon-2019-0389.
Karakaya S, Karadağ İ, Yılmaz ME, Çakmak Öksüzoğlu ÖB. High neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and low lymphocyte levels are correlated with worse pathological complete response rates. Cureus. 2022;14(3): e22972. https://doi.org/10.7759/cureus.22972.
Ramsay G, Ritchie DT, MacKay C, Parnaby C, Murray G, Samuel L. Can Haematology Blood tests at time of diagnosis predict response to neoadjuvant treatment in locally advanced rectal cancer? Dig Surg. 2019;36(6):495–501. https://doi.org/10.1159/000493433.
Bedin C, Crotti S, D’Angelo E, D’Aronco S, Pucciarelli S, Agostini M. Circulating Biomarkers for response prediction of rectal cancer to neoadjuvant chemoradiotherapy. CMC. 2020;27(25):4274–94. https://doi.org/10.2174/0929867326666190507084839.
Morais M, Pinto DM, Machado JC, Carneiro S. ctDNA on liquid biopsy for predicting response and prognosis in locally advanced rectal cancer: a systematic review. Eur J Surg Oncol. 2022;48(1):218–27. https://doi.org/10.1016/j.ejso.2021.08.034.
Author information
Authors and Affiliations
Contributions
Conceptualization: Cindy Kin. Literature search: Cintia Kimura and Eliza Crowder. Analysis, writing, critical revisions: Cindy Kin, Cintia Kimura, and Eliza Crowder. Figures and Tables: Cindy Kin.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kimura, C., Crowder, S.E. & Kin, C. Is It Really Gone? Assessing Response to Neoadjuvant Therapy in Rectal Cancer. J Gastrointest Canc 54, 703–711 (2023). https://doi.org/10.1007/s12029-022-00889-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00889-x