Abstract
Background
Gastric cancer (GC) is one of the leading causes of cancer-related death worldwide. The first-line treatment for GC is a combination of platinum and fluoropyrimidine-based therapy. Based on the positive results of RAINBOW and REGARD trials, ramucirumab either alone or in combination with paclitaxel has proved to be a safe and active option for second-line treatment in GC patients.
Material and methods
Advanced GC patients who received a 28-day cycles of ramucirumab and paclitaxel until disease progression or unacceptable toxicity were evaluated. Eligible patients had ECOG PS ≤ 1 and adequate organ function. Baseline characteristics were assessed for progression-free survival (PFS) and overall survival (OS). The Kaplan–Meier method and Cox proportional-hazards regression models were used for survival analyses.
Results
In our single institution experience, we included a total of 67 patients. A median OS of 8 months and a median PFS of 4 months, were recorded. In patients experiencing an initial partial response (PR), we observed a significant association between tumor response and survival outcomes (OS and PFS). The OS and PFS were 15 and 11 months in patients who experienced PR compared to 8 and 4 months in patients without PR (p = 0.02; p = 0.04).
Conclusion
Treatment with ramucirumab plus paclitaxel yielded the highest overall response rate reported to date for patients with previously treated advanced GC. In our experience, the initial tumor response is associated with a greater survival benefit which could be further improved by the identification of biomarkers predicting response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer death worldwide [1]. The high mortality rate in GC is mainly due to the lack of specific early manifestations, subsequently leading to the late diagnosis and treatment [2, 3].
For patients with advanced or recurrent GC combination chemotherapy regimens consisting of fluoropyrimidines and platinum, with trastuzumab, in HER2 positive cancer, or a third agent such as taxane or anthracycline are the gold standard in the first line treatment [4,5,6,7,8]. However, the prognosis for these patients remains poor, with a median overall survival (OS) that does not exceed 10–12 months [9, 10]. Furthermore, the majority of patients do not respond or relapse within a short time after the end of first-line therapy and only about one-third of patients receive second-line chemotherapy treatments [11]. Various cytotoxic agents both as monotherapy and in combination have been extensively studied in the second-line setting with minimal benefit in these patients [12, 13].
However, although clinical studies have shown that second-line therapy improves OS compared to BSC to a statistically significant extent in actual clinical practice, second-line regimen may not be offered to all patients, mainly due to poor PS experienced after first-line therapy [14,15,16]. Furthermore, in translating the survival benefit from clinical trials to real life, regional ethnic differences must be considered. In fact, almost all Asian patients with metastatic GC receive second-line therapy while in Western countries, less than half of patients receive progressive second-line treatment after first-line therapy [17].
More recently, the use of ramucirumab, a human monoclonal antibody (IgG1) vascular endothelial growth factor receptor-2 (VEGFR-2) antagonist, plus paclitaxel versus paclitaxel alone in second line showed to increase median OS (9.6 months vs 7.4 months) and progression free survival (PSF) (4.4 months vs 2.9 months) in all subgroups of the phase III (RAINBOW) trial [18]. Ramucirumab plus paclitaxel demonstrated the highest objective response rate (ORR) of 28% reported in the second line setting compared with 16% in the paclitaxel alone group (p = 0.0001). Ramucirumab alone versus best supportive care (BSC) confirmed to improve OS in the REGARD study [19]. Based on these findings, paclitaxel plus ramucirumab became the standard second-line treatment for advanced GC. In this study, we retrospectively evaluated the efficacy of ramucirumab plus paclitaxel in patients with advanced GC, presenting, details of the association between partial tumor response and survival outcomes.
Material and Methods
Patients’ Population
We examined the medical records of patients with metastatic GC who received a second-line therapy with ramucirumab and paclitaxel in a single institution from January 2016 to April 2021.
Eligibility criteria for treatment with ramucirumab and paclitaxel included the following: presence of histologically confirmed metastatic gastric cancer; Eastern Cooperative Oncology Group Performance Status (ECOG PS) of ≤ 2; adequate bone marrow, renal and liver function (including, absolute neutrophil count ≥ 1.5 × 109L, platelet count ≥ 100,000/mm3, and hemoglobin level ≥ 9 g/dl; estimate glomerular filtrate rate ≥ 60 ml/min bilirubin level ≤ the upper limit of normal). The exceptions related to enzymatic alterations (e.g., Gilbert Syndrome) or values of Hb < 9 g/dl from gastric bleeding, were considered individually. Patients received previous first-line treatment for metastatic disease and at least one course of therapy with ramucirumab plus paclitaxel have been considered for our study.
Treatment Schedule
Patients received a 28-day cycle of ramucirumab (8 mg/kg intravenously) administered on days 1 and 15 and paclitaxel (80 mg/m2 intravenously) on days 1, 8, and 15. The treatment was continued until disease progression or unacceptable toxicity. The dose reduction of drugs at starting the treatment was agreed depending on ECOG PS, comorbidities or toxicities from previous treatments. Dose modification and interruption of treatment were performed in relation to the criteria established in the pivotal clinical studies [18].
Response Evaluation and Statistical Analysis
Computed tomography scans were performed every 12 weeks or before is clinically indicated. Tumor response was assessed in accordance with RECIST 1.1 [20]. PFS was defined as the interval from the start of treatment with ramucirumab and paclitaxel to the evidence of progressive disease (PD) or death from any cause. Overall survival represents the duration of patient survival from the time of treatment initiation. The best overall response is the best response recorded from initiation of treatment up to disease progression/relapse and may be complete response (CR), partial response (PR), stable disease (SD) or PD. For this analysis, patients have been dichotomized in two groups: patients that achieved PR and those that do not achieved PR (SD and/or PD). The Kaplan–Meier method and Cox proportional hazards regression models were used for survival analyses. Hazard ratio (HR) together with 95% confidence interval (CI) were provided for Cox proportional hazards regression analyses. A two-sided p-value < 0.05 was considered statistically significant. Statistical analyses were performed using STATA v.2012. This study was approved by the Comitato Etico Regionale for clinical experimentation of Toscana region (Italy) Area Vasta Centro section, number: 14912_oss.
Results
Patient’s Characteristics
A total of 67 patients with metastatic GC treated with ramucirumab and paclitaxel between January 2016 and April 2021 were included in our single institution experience. The mean age was 66 years (range, 33–80) with 32.8% more than 70 years; ECOG-PS was 1 in 34 (50.7%) of patients. All patients had previously received a treatment with platinum and fluoropyrimidine-based chemotherapy regimens, and 40 patients (59.7%) had received previous surgery. Thirty-nine (58.2%) patients experienced time to disease on first-line therapy < 6 months. The number of metastatic sites was ≥ 3 in 21 (31.3%) patients and peritoneal involvement was equally present in 21 (31.3%) patients. Patient characteristics are shown in Table 1.
Treatment Results
The median cycles received were 4 (range, 1–24). The number of patients who required a reduced dose of ramucirumab or paclitaxel was 11 (16.4%) and 45 (67.2%), respectively. Treatment was equally delayed for the two drugs. Twenty-six patients discontinued ramucirumab and 29 (43.3%) patients discontinued paclitaxel due to disease progression, toxicity, or other reasons. Thirty-three (49.2%) patients received a subsequent line of chemotherapy (Table 2). A median OS of 8 months (range, 7–10) and a median PFS of 4 months (range, 3–5) were recorded (Table 3).
Correlation Between Response Tumor and Survival Parameters
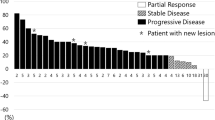
We focused the analysis on patients with clinical benefit that is PR (no CRs were observed) at least at the first disease reassessment. Clinical and treatment characteristics of patients who experienced PR (N = 7) and of those who have not reached PR (SD + PD, N = 60) are shown in Table 1. Patients in PR group received a median of 10 [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22] cycles of chemotherapy compared to 4 [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24] cycles in patients without PR (p = 0.01). No statistical differences were observed concerning dose reduction, treatment delayed and treatment interruption between the two groups; all patients with PR received a subsequent line of chemotherapy, contrary to 43.3% of patients without PR (Table 2). In this study, we explored the association of survival outcomes with PR observing a significant correlation with both OS and PSF. Specifically, PR was associated with better OS (HR 0.42, 95% CI 0.19–0.94; p = 0.02) and PFS (HR 0.43, 95% CI 0.18–0.98; p = 0.04) compared to non-PR (Figs. 1 and 2). To allow for better interpretation of the data, characteristic of patients experienced PD at first tumor reassessment were separately reported (Table 1 Supplementary information).
Univariate and Multivariate Analysis
Cox regression analysis has been performed on the entire cohort of 67 patients to assess the associations between clinical-pathological variables of interest and better survival outcomes. Risk variables assessed included age, gender, tumor location, number of metastatic sites, previous surgery, ECOG PS, peritoneal metastases, time to progressive disease on first-line therapy and partial response.
In the univariate analysis, ECOG PS = 1 (versus 0) (HR, 1.44; 95% CI, 1.05–1.93; p = 0.02), presence of peritoneal metastases (HR, 1.83; 95% CI, 1.10–2.35; p = 0.03), time to progressive disease on first-line therapy < 6 months (HR, 1.44; 95% CI, 1.21–1.80; p = 0.03) and previous surgery (HR, 1.10; 95% CI, 1.05–1.77; p = 0.04) were correlated with worse survival, whereas PR (HR, 0.43; 95% CI, 0.18–0.98; p = 0.04) has been correlated with better survival outcomes.
Multivariate analysis confirmed ECOG PS (HR, 1.24; 95% CI, 1.01–1.76; p = 0.04) and peritoneal metastases (HR, 2.03; 95% CI, 0.403.68; p < 0.01) as negative prognostic factors for survival and PR (HR, 0.55; 95% CI, 0.44–0.927; p = 0.04) as prognostic positive variable.
Discussion
A large part of patients with GC are initially diagnosed with unresectable or metastatic disease, and first-line chemotherapy guarantees a median overall survival often not exceeding 12 months [21, 22].
To date, the RAINBOW trial demonstrated the highest second-line response rate in patients with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma. Significant improvements in PFS (HR 0.635; 95%CI 0.536, 0.752; p < 0.0001), OS (HR 0.807; 95% CI 0.678–0.962; p = 0.0169), and overall response rates (27.9% p = 0.0001) have been reached [18]. Moreover, a subgroup analysis in Western population, showed a median OS of 8.5 months for ramucirumab and paclitaxel and 5.9 months for paclitaxel alone. Median PFS was 4.2 and 2.8 months in the two group, respectively with an ORR of 26.8% in the combination arm (p = 0.0004) [23]. Subsequently, real-life studies on ramucirumab and paclitaxel as second-line therapy in metastatic GC have achieved results in terms of OS and PSF overlapping [24, 25].
In our single institution study, median OS [8 months, range 7–10] and PFS [4 months, range 3–5] were in line with the pivotal trial. However, focusing on patients who achieved PR to treatment, a define benefit was recorded in terms of survival. In these patients, compared with patients that do not experienced PR, OS and PFS were 15 months (HR 0.43; 95% CI 0.18–0.98; p = 0.04) and 11 months (HR 0.42; 95% CI 0.19–0.94; p = 0.02), respectively (Figs. 1 and 2). At the multivariate analysis, we identified ECOG PS = 1 (HR, 1.24; 95% CI, 1.01–1.76; p = 0.04) and presence of peritoneal metastases (HR, 2.03; 95% CI, 0.403.68; p < 0.01) as factors correlated with worse survival outcomes, whereas PR (HR, 0.55; 95% CI, 0.44–0.927; p = 0.04) has been correlated with higher survival. Thus, although second-line treatments have guaranteed an improvement in terms of survival, the prognosis of these patients remains poor and only a few benefit from it. This emphasizes the need for identifying predictive biomarkers to better select patient and direct it to second-line chemotherapy with ramucirumab and paclitaxel or clinical trials.
Previously, depth of response (DpR) has been correlated with post progression survival in subgroups of gastric cancer patients receiving second-line chemotherapy, indicating DpR as possible new predictor for efficiency [26]. However, the predictive value of DpR is not sure, which may be related to other factors, such us the mutation status of Human Epidermal Growth Factor Receptor 2 and treatment methods, among others.
A recent prospective study has suggested the prognostic value of some circulating factors such as neutrophil-to-lymphocyte ratio (NLR) and myeloid-derived suppressor cells (MDSCs) on survival outcomes in GC patients receiving ramucirumab plus paclitaxel treatment [27]. Another study suggested that the occurrence of high-grade neutropenia can predict response to treatment with ramucirumab and paclitaxel. In this analysis, patients who experienced grade ≥ 3 neutropenia had a PFS of 6.6 months (95% CI 3.3–8.4) and an OS of 11 months (95% CI 5.9–13.1) compared to 4.4 months (95% CI 3.9–5.2) and 8.7 months (95% CI 7.8–10.1) for patients with lower grade neutropenia [28].
Natsume et al. identified a correlation between aberrant expression of placental growth factor (PlGF) and ramucirumab responders and non-responders. OS (p = 0.046) and PFS (p = 0.016) were significantly shorter in the PlGF-high group than in the PlGF-low group. Overall response rates were 50% and 0% in the PlGF-low and high group, respectively [29].
However, despite the various efforts made, no predictive biomarkers have yet been identified and the mechanism underlying the response or resistance to the combination of ramucirumab and paclitaxel remains unclear [30].
Moreover, Cascinu et al. reported the correlation between tumor response, and the symptom palliation in the intent to treat population of the RAINBOW study, as also observed in patients with metastatic breast cancer receiving chemotherapy [31].
This study presents several limitations mainly due to the retrospective nature of the data collection, the limited number of patients included, and a single Oncologic Center involved. Moreover, the inclusion of patients with primary progressive disease, who have an extremely poor survival, in the subgroup which do not experience PR, amplifies the prognostic impact of PR itself. However, while aware that tumor response should be associated with improved survival, this may not necessarily occur [32]. What we want to underline with this work is the statistically significant difference in OS and PFS that we observed between the patients who have achieved PR and who have not achieved PR, which further pushes us to continue looking for biomarkers capable of selecting patients guaranteeing the best therapeutic choice Figs. 1 and 2.
Conclusion
Second-line treatment with ramucirumab plus paclitaxel is the currently recognized standard of care for patients with advanced gastric adenocarcinoma or GEJ previously treated with recommended first-line therapy. Although with many limitations, we have reported statistically significant survival benefits in patients who exhibit a partial response as the best response to treatment. Since the prognosis of these patients remains very limited, the identification of predictive biomarkers of response could improve the selection of patients who benefit most from this association.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Canc J Clin. 2021;71:209–249. https://doi.org/10.3322/caac.21660.
Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10–27. https://doi.org/10.14740/wjon1166.
Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumor Biol 2017;39: https://doi.org/10.1177/1010428317714626.
Cheng J, Cai M, Shuai X, Gao J, Wang G, Tao K. First-line systemic therapy for advanced gastric cancer: a systematic review and network meta-analysis. Therap Adv Med Oncol. 2019;11: https://doi.org/10.1177/1758835919877726.
Roviello G, Aprile G, D’Angelo A, Iannone LF, Roviello F, Polom K, et al. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: where do we stand? Gastric Cancer. 2021;24:765–79. https://doi.org/10.1007/s10120-021-01182-9.
Cheng J, Cai M, Shuai X, Gao J, Wang G, Tao K. First-line systemic therapy for advanced gastric cancer: a systematic review and network meta-analysis. Therapeutic advances in medical oncology. 2019;11:1758835919877726. https://doi.org/10.1177/1758835919877726.
Petrioli R, Francini E, Roviello F, Marrelli D, Fiaschi AI, Laera L, et al. Sequential treatment with epirubicin, oxaliplatin and 5FU (EOF) followed by docetaxel, oxaliplatin and 5FU (DOF) in patients with advanced gastric or gastroesophageal cancer: a single-institution experience. Cancer Chemother Pharmacol. 2015;75:941–7. https://doi.org/10.1007/s00280-015-2715-x.
Available online at NCCN.org/patients NCCN GUIDELINES FOR PATIENTS ® Stomach Cancer FOUNDATION Guiding Treatment. Changing Lives. National Comprehensive Cancer Network. 2021.
Zaanan A, Bouché O, Benhaim L, Buecher B, Chapelle N, Dubreuil O, et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis. 2018;50:768–79. https://doi.org/10.1016/j.dld.2018.04.025.
Gastric Cancer Treatment Recommendations. Available at: https://www.esmo.org/guidelines/guidelines-by-topic/gastrointestinal-cancers/gastric-cancer/eupdate-gastric-cancer-treatment-recommendations. [Accessed 24 Aug 2022].
Review of second-line chemotherapy for advanced gastric adenocarcinoma - PubMed. Available at: https://pubmed.ncbi.nlm.nih.gov/15830569/. [Accessed 17 Nov 2021].
Kanagavel D, Fedyanin M, Tryakin A, Tjulandin S. Second-line treatment of metastatic gastric cancer: current options and future directions. World J Gastroenterol. 2015;21:11621–35. https://doi.org/10.3748/wjg.v21.i41.11621.
Roviello G, Catalano M, D’Angelo A, Palmieri VE. Second line of treatment for HER2-positive gastric cancer: an evolving issue. Rep Pract Oncol Radiother. 2021;26:316–7. https://doi.org/10.5603/RPOR.a2021.0024.
Catalano V, Graziano F, Santini D, D’Emidio S, Baldelli AM, Rossi D, et al. Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer. 2008;99:1402–7. https://doi.org/10.1038/sj.bjc.6604732.
Roviello F, Marrelli D, De Manzoni G, Morgagni P, Di Leo A, Saragoni L, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90:1113–9. https://doi.org/10.1002/bjs.4164.
Kanagavel D, Fedyanin M, Tryakin A, Tjulandin S. Second-line treatment of metastatic gastric cancer: current options and future directions. World J Gastroenterol. 2015;21:11621–35. https://doi.org/10.3748/wjg.v21.i41.11621.
Kim J, Sun CL, Mailey B, Prendergast C, Artinyan A, Bhatia S, et al. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol. 2009;21:152–60. https://doi.org/10.1093/annonc/mdp290.
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. https://doi.org/10.1016/S1470-2045(14)70420-6.
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet. 2014;383:31–9. https://doi.org/10.1016/S0140-6736(13)61719-5.
Schwartz LH, Litière S, De Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1 - Update and clarification: from the RECIST committee. Euro J Canc. 2016;62:132–137. https://doi.org/10.1016/j.ejca.2016.03.081.
Zaanan A, Bouché O, Benhaim L, Buecher B, Chapelle N, Dubreuil O, et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig Liver Dis. 2018;50:768–79. https://doi.org/10.1016/j.dld.2018.04.025.
Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up - PubMed. Available at: https://pubmed.ncbi.nlm.nih.gov/27664260/. [Accessed 17 Nov 2021].
Shitara K, Muro K, Shimada Y, Hironaka S, Sugimoto N, Komatsu Y, et al. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer. 2016;19:927–38. https://doi.org/10.1007/s10120-015-0559-z.
Matsumoto H, Kawazoe A, Shimada K, Fukuoka S, Kuboki Y, Bando H, et al. A retrospective study of the safety and efficacy of paclitaxel plus ramucirumab in patients with advanced or recurrent gastric cancer with ascites. BMC Cancer. 2018;18: https://doi.org/10.1186/s12885-018-4057-7.
Garrido M. The safety and efficacy of ramucirumab in combination with paclitaxel for the treatment of advanced gastric or gastro-esophageal junction adenocarcinoma. Expert Rev Anticancer Ther. 2016;16:1005–10. https://doi.org/10.1080/14737140.2016.1231576.
Lee C kun, Kim S seob, Park S, Kim C, Heo SJ, Lim JS, et al. Depth of response is a significant predictor for long-term outcome in advanced gastric cancer patients treated with trastuzumab. Oncotarget. 2017;8:31169–31179. https://doi.org/10.18632/oncotarget.16099.
Kim H-D, Ryu M-H, Yoon S, Na Y-S, Moon M, Lee H, et al. Clinical implications of neutrophil-to-lymphocyte ratio and MDSC kinetics in gastric cancer patients treated with ramucirumab plus paclitaxel. Chinese J Canc Res. 2020;32:621–630. https://doi.org/10.21147/j.issn.1000-9604.2020.05.07.
Roviello G, Conca R, D’Angelo A, Multari AG, Paganini G, Chiriacò G, et al. Association between neutropenia and response to ramucirumab and paclitaxel in patients with metastatic gastric cancer. Anticancer Drugs. 2020;31:632–6. https://doi.org/10.1097/CAD.0000000000000905.
Natsume M, Shimura T, Iwasaki H, Okuda Y, Kitagawa M, Okamoto Y, et al. Placental growth factor is a predictive biomarker for ramucirumab treatment in advanced gastric cancer. Cancer Chemother Pharmacol. 2019;83:1037–46. https://doi.org/10.1007/s00280-019-03817-2.
Van Cutsem E, Muro K, Cunningham D, Bodoky G, Sobrero A, Cascinu S, et al. Biomarker analyses of second-line ramucirumab in patients with advanced gastric cancer from RAINBOW, a global, randomized, double-blind, phase 3 study. Eur J Cancer. 2020;127:150–7. https://doi.org/10.1016/j.ejca.2019.10.026.
Cascinu S, Bodoky G, Muro K, Van Cutsem E, Oh SC, Folprecht G, et al. Tumor response and symptom palliation from RAINBOW, a Phase III trial of ramucirumab plus paclitaxel in previously treated advanced gastric cancer. Oncologist. 2021;26:e414–24. https://doi.org/10.1002/onco.13623.
Markman M. What does tumor shrinkage mean to the patient receiving chemotherapy? Clevel Clin J Med. 1996;63:301–2. https://doi.org/10.3949/ccjm.63.5.301.
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
GR had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: GR Acquisition of data: EB, GR, FP, ID, CW Recruiting patients: GR, LA.Analysis and interpretation of data: GR. Drafting of the manuscript: MC, RG. Critical revision of the manuscript for important intellectual content: GR,LA. Statistical analysis: GR. Obtaining funding: None. Administrative, technical, or material support: None. Supervision: GR. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roviello, G., Martina, C., Winchler, C. et al. Correlation Between Tumor Response and Survival Outcomes in Patients with Advanced Gastric Cancer Receiving Ramucirumab and Paclitaxel as Second-Line Therapy. J Gastrointest Canc 54, 802–808 (2023). https://doi.org/10.1007/s12029-022-00865-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00865-5