Abstract
Introduction
Gall bladder cancer (GBC) has high prevalence in the Indo-Gangetic belt in India. While the first-line chemotherapy (CT1) has been established as gemcitabine–platinum doublet in advanced GBC, there is no standard recommendation or guidelines regarding feasibility of second-line therapy.
Methods
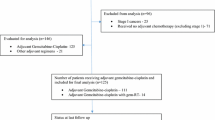
We performed a retrospective analysis of all patients who received second-line of chemotherapy (CT2) at our institution from July 2012 to December 2014. Patient records were examined for efficacy and toxicity of administered CT2, along with response rates (RR) and survival. Potential prognostic factors were also evaluated.
Results
Eighty-seven patients received CT2 in the predefined period. Ninety-nine percent of patients had received a gemcitabine-based regimen as CT1 with a median progression-free survival (PFS) of 5 months before CT2. 51.7 % patients had undergone surgery prior with 5.7 % patients having received radiotherapy previously. Prior to beginning CT2, PS was 0/1 in 67.8 % patients, albumin was >4 g% in 40.2 % and CA 19.9 was raised in a majority (66.7 %) patients, respectively. As per institution protocol, a majority of patients (89.6 %) were administered CAP-IRI regimen. Overall RR and disease control rates (DCR) were 21.8 % and 41.3 %, respectively. Median progression-free survival (PFS) and overall survival (OS) were 6 and 8 months, with no significant differences between CAP-IRI and other regimens. Adverse effects were tolerable, with dose reduced upfront in 23 % patients and 11.5 % patients during subsequent cycles of CT. ECOG Performance Status (PS) of 0/1 was a significant prognostic variable for OS on multivariate analysis (p = 0.003).
Conclusion
CAP-IRI is a well-tolerated second-line chemotherapeutic regimen in patients with advanced GBC. Careful selection of patients is required when administering second-line chemotherapy to advanced GBC patients, with particular emphasis on ECOG PS.



Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F et al.- GLOBOCAN 2008, Cancer Incidence and Mortality International Agency for Research on Cancer; 2010. IARC Cancer Base No 10.
Murthy NS. Trends and patterns of cancer load in India in epidemiological estimation and analysis, mimeographed, submitted to Indian Council of Medical Research (ICMR), New Delhi, India. 2009.
Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–65.
Saetta AA, Papanastasiou P, Michalopoulos NV, et al. Mutational analysis of BRAF in gallbladder carcinomas in association with K-ras and p53 mutations and microsatellite instability. Virchows Arch. 2004;445:179–82.
Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–12.
Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902.
Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689–700.
Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28:4581–6.
Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81.
Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469–74.
Sasaki T, Isayama H, Nakai Y, Mizuno S, Yamamoto K, Yagioka H, et al. Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs. 2011;29(6):1488–93.
Oh SY, Jeong CY, Hong SC, Kim TH, Ha CY, Kim HJ, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs. 2011;29(5):1066–72.
Sasaki T, Isayama H, Yashima Y, Yagioka H, Kogure H, Arizumi T, et al. S-1 monotherapy in patients with advanced biliary tract cancer. Oncology. 2009;77(1):71–4.
Suzuki E, Ikeda M, Okusaka T, Nakamori S, Ohkawa S, Nagakawa T et al. A multicenter phase II study of S-1 for gemcitabine-refractory biliary tract cancer. Cancer Chemother Pharmacol 2013.
Sasaki T, Isayama H, Nakai Y, Mizuno S, Yamamoto K, Yagioka H, et al. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs. 2012;30(2):708–13.
Lamarca A, Hubner RA, David Ryder W. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328–38.
Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–602.
Cassier PA, Thevenet C, Walter T, et al. Outcome of patients receiving chemotherapy for advanced biliary tract or gallbladder carcinoma. Eur J Gastroenterol Hepatol. 2010;22:1111–7.
Kobayashi K, Tsuji A, Morita S, et al. A phase II study of LFP therapy (5-FU (5-fluorourasil) continuous infusion (CVI) and Low-dose consecutive (Cisplatin) CDDP) in advanced biliary tract carcinoma. BMC Cancer. 2006;6:121.
Lim KH, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Outcome of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) chemotherapy and analysis of prognostic factors in patients with refractory advanced biliary tract cancer. Oncology. 2012;83(2):57–66.
He S, Shen J, Sun X, Liu L, Dong J. A phase II FOLFOX-4 regimen as second-line treatment in advanced biliary tract cancer refractory to gemcitabine/cisplatin. J Chemother. 2014;26(4):243–47.
Sasaki T, Isayama H, Nakai Y, Takahara N, Satoh Y, Takai D, et al. A pilot study of salvage irinotecan monotherapy for advanced biliary tract cancer. Anticancer Res. 2013;33(6):2619–22.
Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19:2282–92.
Twelves C, Boyer M, Findlay M, Cassidy J, Weitzel C, Barker C, et al. Capecitabine (Xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a phase III trial conducted in patients with advanced colorectal carcinoma. Eur J Cancer (Oxford, England: 1990). 2001;37:597–604.
Pino MS, Milella M, Gelibter A. Capecitabine and celecoxib as second-line treatment of advanced pancreatic and biliary tract cancers. Oncology. 2009;76(4):254–61.
Walter T, Horgan AM, Mcnamara M. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer. 2013;49(2):329–35.
G.Brandi et al. Second-line chemotherapy in patients with biliary tract cancer. J Clin Oncol 29: 2011 (suppl; abstr e14590) 2013.
Shirasaka T, Shimamato Y, Ohshimo H, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57.
Katayose Y, Ohtsuka H, Kitamura Y. An analysis of a second-line S-1 monotherapy for gemcitabine-refractory biliary tract cancer. Hepatogastroenterol. 2012;59(115):691–5.
Park I, Lee JL, Ryu MH. Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first-line palliative chemotherapy. Cancer. 2009;115(18):4148–55.
Fornaro L, Cereda S, Aprile G. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer. 2014;110(9):2165–9.
Acknowledgments
No fundings received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
None to declare.
Additional information
Key Message:
In gall bladder cancers, with the use of second-line chemotherapy like CAP-IRI, the ORR, DCR, Median PFS and OS were 21.8 %, 41.3 %, 6 months and 8 months respectively. Adverse effects were tolerable, with dose reduction upfront and in subsequent cycles were 23 and 11.5 % patients respectively. ECOG PS of 0/1 was a significant prognostic variable for OS on multivariate analysis (p = 0.003).
This is a first study which concentrates only on gall bladder cancers and here we introduce CAP-IRI, a safe and effective option in second line with selection of patients based on ECOG PS. As we see approximately 600 gall bladder cancers per year, it can form a base for considering second-line chemotherapy in patients with good ECOG PS as well as a future randomized Phase 3 trial comparing this regimen with best supportive care.
Rights and permissions
About this article
Cite this article
Ramaswamy, A., Ostwal, V., Pande, N. et al. Second-Line Palliative Chemotherapy in Advanced Gall Bladder Cancer, CAP-IRI: Safe and Effective Option. J Gastrointest Canc 47, 305–312 (2016). https://doi.org/10.1007/s12029-016-9828-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-016-9828-2