Abstract
Background
This study investigated trajectory profiles and the association of concentrations of the biomarkers neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) in ventricular cerebrospinal fluid (CSF) with clinical outcome at 1 year and 10–15 years after a severe traumatic brain injury (sTBI).
Methods
This study included patients with sTBI at the Neurointensive Care Unit at Sahlgrenska University Hospital, Gothenburg, Sweden. The injury was regarded as severe if patients had a Glasgow Coma Scale ≤ 8 corresponding to Reaction Level Scale ≥ 4. CSF was collected from a ventricular catheter during a 2-week period. Concentrations of NfL and GFAP in CSF were analyzed with enzyme-linked immunosorbent assay. The Glasgow Outcome Scale (GOS) was used to assess the 1-year and 10–15-year outcomes. After adjustment for age and previous neurological diseases, logistic regression was performed for the outcomes GOS 1 (dead) or GOS 2–5 (alive) and GOS 1–3 (poor) or GOS 4–5 (good) versus the independent continuous variables (NfL and GFAP).
Results
Fifty-three patients with sTBI were investigated; forty-seven adults are presented in the article, and six children (aged 7–18 years) are described in Supplement 1. The CSF concentrations of NfL gradually increased over 2 weeks post trauma, whereas GFAP concentrations peaked on days 3–4. Increasing NfL and GFAP CSF concentrations increased the odds of GOS 1–3 outcome 1 year after trauma (odds ratio [OR] 1.73, 95% confidence interval [CI] 1.07–2.80, p = 0.025; and OR 1.61, 95% CI 1.09–2.37, p = 0.016, respectively). Similarly, increasing CSF concentrations of NfL and GFAP increased the odds for GOS 1–3 outcome 10–15 years after trauma (OR 2.04, 95% CI 1.05–3.96, p = 0.035; and OR 1.60, 95% CI 1.02–2.00, p = 0.040).
Conclusions
This study shows that initial high concentrations of NfL and GFAP in CSF are both associated with higher odds for GOS 1–3 outcome 1 year and 10–15 years after an sTBI, implicating its potential usage as a prognostic marker in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe traumatic brain injury (sTBI) (i.e., Glasgow Coma Scale [GCS] of ≤ 8) is a condition that includes focal contusions, intradural and extradural hematomas, and diffuse axonal injuries [1]. These injuries often lead to severe lifelong neurological deficits or even death. sTBI is associated with changes in the levels of biomarkers in cerebrospinal fluid (CSF), indicating different pathology and brain cell origin [2, 3].
In neurons, neurofilaments, including neurofilament light (NfL), are found in axons and are involved in axonal transport, axonal growth, and cytoskeleton plasticity [3, 4]. When neurons are exposed to trauma, intracellular damage leads to axonal swelling and disconnection and the NfL passes into the CSF [5]. Glial fibrillary acidic protein (GFAP) is expressed by astrocytes, and when injured, GFAP is released into the CSF [2]. Further, as a response to trauma, GFAP synthesis increases, contributing to the extension and thickening of the astrocytic processes observed around damaged areas. Astrocytes provide both functional and structural support to neurons and the blood–brain barrier (BBB) [2].
Previously, parts of this sTBI population have been investigated to relate GFAP, S100b, and NfL in serum as well as β-amyloid, amyloid precursor protein, and tau in CSF and the gene APOE ε4 with 1-year outcome [6,7,8,9,10,11]. CSF NfL was analyzed by Shahim et al. [6] and compared with serum NfL levels but was never related to outcome.
In the present study, we analyzed NfL, a biomarker for of axonal injury, and GFAP, a biomarker of astrocyte injury and response, in ventricular CSF [2, 3]. We explored the trajectory profiles of NfL and GFAP in the CSF during the initial 2 weeks in patients with sTBI. Further, our hypothesis was that concentrations of NfL and GFAP in the CSF analyzed early after an sTBI were able to predict 1-year and 10–15-year patient outcome.
Methods
Patients
The study was performed in accordance with the provisions of the Helsinki Declaration. The University Hospital Medical Ethics Committee, Gothenburg, Sweden, approved the initial study protocol (S-161 00) as well as a supplementary application for the secondary assessment (438-15). The investigation was registered on ClinicalTrials.gov (identifier NCT05138692). Informed written consent was obtained from each patient or next of kin.
Patients with an sTBI were included between October 2000 and November 2004. All the included patients had been admitted to the neurointensive care unit (NICU) at Sahlgrenska University Hospital within 48 h after the insult. The injury was regarded as severe (sTBI) if patients were unconscious, the GCS [12] was ≤ 8, or the Reaction Level Scale [13] was ≥ 4, corresponding to a GCS ≤ 8. In addition, the following inclusion criteria had to be fulfilled: (1) need for artificial ventilation, (2) need for insertion of a ventricular catheter for intracranial pressure (ICP) monitoring, and (3) residing in Sweden for follow-up. Finally, both children (> 7 years) and adults were included. The pediatric population is presented separately in Supplement 1.
After a clinical and radiologic evaluation using computed tomography (CT), patients received a ventricular catheter for ICP monitoring and, if needed, therapeutic CSF drainage. Patients were treated according to a standardized protocol, the Lund concept [14]. The Lund concept is a therapy with the main goal to decrease ICP and improve microcirculation. It is ICP driven, not cerebral perfusion pressure (CPP) driven, with a goal of having an ICP of < 20 mm Hg (27cmH2O), concomitant with a CPP > 50 mm Hg. This is achieved by keeping colloid pressure high (albumin) combined with normalized electrolytes, diuretics, heavy sedation, temperature regulation, and body positioning [14]. If ICP and CPP could not be kept within decided limits and/or an eminent herniation was at hand, hematomas were surgically evacuated and/or decompressive hemicraniectomy was performed. Physiologic and laboratory variables were continuously monitored and adjusted to be kept within predefined limits, described in previously published investigations [6,7,8,9,10,11, 15].
Assessments
CSF samples were collected intermittently on days 0 (trauma day), 1–4, 6, and 8 and once during days 11–18. Because of the risk of herniation, some CSF samples were not obtained because of high ICP, decided by the neurosurgeon in charge. CSF samples were aliquoted and then frozen at − 70 °C until analyzed. Concentrations of NfL and GFAP in the CSF were measured using enzyme-linked immunosorbent assays (ELISAs), Uman Diagnostics ELISA for NfL and Rosengrens in-house ELISA for GFAP [16, 17]. Within-run and between-run variability have a coefficient of variability (CV) < 10%. The analyses were performed in 2006.
Data on previous diseases, type of injury, operations, and rehab were mainly collected from medical records. The Marshall classification was used to categorize the damage seen on initial CT scans and was assessed by neuroradiologists [18].
A neurologist blinded to biomarker concentrations performed the first clinical assessment at 1 year post injury (2001–2006). The outcome was assessed using the extended eight-graded version of the Glasgow Outcome Scale (GOS) and was then converted to the five-graded version: GOS 1 = dead, GOS 2 = vegetative state, GOS 3 = severe disability, GOS 4 = moderate disability, and GOS 5 = good recovery. The GOS was dichotomized into GOS 1–3 (poor) or GOS 4–5 (good) outcome and then further into GOS 1 (dead) or GOS 2–5 (alive) [19, 20]. King’s Outcome Scale for Childhood Head Injury was used to assess outcome in children 1 year after trauma [21].
For the second assessment, 10–15 years post trauma, patients were checked against the Swedish Tax Agency and medical records to retrieve information about whether they were still alive. In 2015, through telephone-based interviews, one investigator, who was different from the first interviewer, conducted the second assessment according to the GOS protocol [19, 20]. If patients were unable to provide sufficient information about their GOS, this information was instead collected from relatives or caregivers.
Statistics
The distributions of skewed continuous variables, such as NfL and GFAP, are given as the median, first quartile, and third quartile in tables and with the addition of minimum and maximum in boxplots. Unadjusted correlations were analyzed with Spearman’s rank correlation (rs), and adjusted correlations were analyzed with Spearman’s partial correlation coefficient. For comparisons of NfL and GFAP between two groups, Fisher’s permutation test was used for unadjusted analyses, and analysis of covariance was used for adjusted analyses, both on log-transformed values to describe the difference between two groups regarding the NfL and GFAP fold change, and the 95% confidence interval (CI) is given, calculated as the antilogarithm of the mean difference with the 95% CI on the log scale. Fold change should be interpreted as the number of times greater or less by which one group is compared with the other. The Mann–Whitney U-test was used to compare the concentration differences in continuous variables (NfL and GFAP) per sample day between GOS 1–3 (poor) and GOS 3–5 (good) outcome and GOS 1 (dead) and GOS 2–5 (alive). The analyses of correlations between NfL and GFAP against the GOS and the comparison between two GOS groups regarding NfL and GFAP were adjusted for confounders. Confounders are the baseline variables that correlated significantly (Spearman correlation test) with both the GOS and the analyzed NfL and/or GFAP values, on the condition that the variable can affect NfL and/or GFAP concentrations in CSF and GOS results. Adjusted logistic regression was based on the dependent variables of GOS 1 or GOS 2–5 and GOS 1–3 or GOS 4–5 outcome versus the independent continuous variables (NfL and GFAP) on the log scale presented as the odds ratio (OR) with the 95% CI, p values, and the area under the receiver operating characteristic curve. The cutoff values and Youden index were calculated on the best sum of sensitivity and specificity. The number of deaths (n = 9) 1 year after trauma was too small to allow for adjustment analysis. When appropriate, 1-year outcome analyses were adjusted for age and 10–15-year outcome analyses were adjusted for age and pretrauma neurological diseases. All analyses of NfL and GFAP were made on the highest concentration of each patient. All significance tests were two-sided and conducted at the 5% significance level. All statistical analyses were performed using SAS system version 9.4.
Results
Patient Characteristics
A total of 124 eligible patients were available for inclusion, whereas 3 patients were excluded because of the lack of a ventricular catheter, 6 patients or their next of kin failed to give informed consent, 1 patient did not reside in Sweden, and 12 patients, after reconsideration, did not fulfill the criteria for an sTBI. Additionally, three patients were excluded at the 10–15-year follow-up because the 1-year data were missing in two patients and one did not reside in Sweden. Further, of the remaining 99 patients, 46 were excluded because of the lack of CSF samples for biomarker analysis. As a result, 53 patients were included. Six patients were children at the time of injury, and this pediatric population is described separately in Supplement 1. The remaining 47 adult patients are presented as follows. They had a mean age on admission of 45.4 years (SD 16.1 years), and 83% were men (Table 1). Results including both the pediatric and adult population are presented in corresponding tables and figures in Supplement 2.
Nine patients died during the first year after trauma; six of those died within 30 days after trauma. In addition, nine patients died before the second assessment 10–15 years after trauma. The remaining group of 29 patients were available for the second assessment performed with patients (n = 22), relatives (n = 2), a caregiver (n = 1), or a patient and caregiver (n = 1). Three patients were not available for the second assessment, but they were alive (GOS 2–5) according to the Swedish Tax Agency and are included in the results of GOS 1 (dead) versus GOS 2–5 (alive).
Most with GOS 2–5 (surviving n = 38) at 1 year had the same GOS 10–15 years after injury (47%, n = 18), whereas 29% (n = 11) declined, 16% (n = 6) improved, and 8% (n = 3) were missing. The GOS category at 10–15 years after trauma within each GOS category at 1 year post trauma is shown in Fig. 1.
Age correlated significantly with GFAP and GOS at 1 year and 10–15 years after trauma. Pretrauma existing neurological diseases (epilepsy, n = 5; transient ischemic attack, n = 1; previous trauma to the head, n = 2) correlated with GOS 10–15 years after trauma (Table 2). All analyses of 1-year outcome are adjusted for age, whereas those relating to the 10–15-year outcome are adjusted for both age and pretrauma existing neurological diseases. However, the number of patients (n = 9) who had GOS 1 (dead) 1 year after trauma did not allow for any adjustment of the regression analysis. No other confounder was found or needed to be adjusted for. Patient rehabilitation correlated significantly with NfL, GFAP, and GOS at 1 year and GOS at 10–15 years. Rehabilitation per se will not affect the acute-phase biomarkers and is thus considered an effect modifier and not a confounder, and as a result, it has not been adjusted for.
Concentration Dynamics of NfL and GFAP in CSF After an sTBI
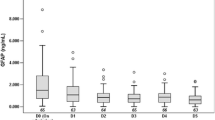
CSF was analyzed on days 0–2 (n = 20), days 3–4 (n = 32), days 6–8 (n = 32), and days 11–18 (n = 13). NfL concentrations gradually increased from days 0–2 to 11–18 post injury, whereas GFAP concentrations peaked on days 3–4, after which they declined (Fig. 2).
Trajectory profile of NfL and GFAP (μg/L) in CSF. Concentrations of NfL and GFAP in the CSF are shown per sample period in boxplots. The median concentrations per sample period are connected to illustrate the trajectory profile of each biomarker. Concentrations of NfL and GFAP are presented in μg/L and on a log10 scale. The sample period is expressed in days after trauma. The numbers of samples were as follows: days 0–2 (n = 20), days 3–4 (n = 32), days 6–8 (n = 32), and days 11–18 (n = 13). CSF cerebral spinal fluid, GFAP glial fibrillary acidic protein, NfL neurofilament light
Initial Maximum CSF Concentrations of NfL and GFAP and Outcome 1 Year After an sTBI: GOS 1–3 (Poor) Versus GOS 4–5 (Good) Outcome
Both NfL and GFAP correlated with 1-year GOS 1–5 (rs = − 0.45, p = 0.001, and rs = − 0.43, p = 0.003; adjusted for age: rs = − 0.42, p = 0.003, and rs = − 0.36, p = 0.013). Concentrations of NfL and GFAP per GOS category 1 year after trauma are shown in Fig. 3a. Levels of NfL and GFAP were significantly higher in patients with GOS 1–3 than in those with GOS 4–5 before and after adjustment for age (Table 3). Patients with GOS 4–5 had (adjusted fold change) 62% lower NfL concentrations and 76% lower GFAP concentrations than patients with GOS 1–3 outcome 1 year after trauma (Table 3). We found significantly higher levels of NfL on days 0–2 and of GFAP on days 0–2 and 11–18 in patients with GOS 1–3 than in patients with GOS 4–5 (Fig. 4a).
Concentrations of NfL and GFAP in each GOS category. Boxplot presenting initial CSF concentrations of NfL and GFAP in each GOS category 1–5. a GOS 1 year after trauma. b GOS 10–15 years after trauma. Each patients maximum CSF concentration of NfL and GFAP was used in the calculation. CSF cerebral spinal fluid, GFAP glial fibrillary acidic protein, GOS Glasgow Outcome Scale, NfL neurofilament light
Concentrations of NfL and GFAP in those with GOS 1–3 (poor) versus those with GOS 4–5 (good outcome) and those with GOS 1 (dead) versus those with GOS 4–5 (alive) 1 year and 10–15 years after trauma. Boxplot presenting initial CSF concentrations of NfL and GFAP (μg/L on a log10 scale), separately, per sample period. Concentrations of NfL and GFAP in CSF, in stratified groups: GOS 1–3 versus GOS 4–5 outcome 1 year after trauma (a) and 10–15 years after trauma (b) and GOS 1 versus GOS 2–5 1 year after trauma (c) and 10–15 years after trauma (d). Each patients maximum CSF concentration of NfL and GFAP was used in the calculation. CSF cerebral spinal fluid, GFAP glial fibrillary acidic protein, GOS Glasgow Outcome Scale, NfL neurofilament light
After age adjustment, a relative increase in NfL and GFAP of 50% significantly increased the odds of GOS 1–3 outcome 1 year after trauma (OR 1.73, p = 0.025, and OR 1.61, p = 0.016, respectively; Fig. 5a). To find cutoff values for the 1-year outcome, we used the Youden index and noted that an NfL concentration of ≥ 12.0 μg/L (Youden index, J = 0.41) separated patients with GOS 1–3 outcome from those with GOS 4–5 outcome with a sensitivity of 0.68 and a specificity of 0.73. Similarly, a GFAP concentration of ≥ 83.6 μg/L (J = 0.53) separated patients with GOS 1–3 outcome from those with GOS 4–5 outcome with a sensitivity of 0.80 and a specificity of 0.73 (Fig. 6a).
The OR for GOS 1–3 (poor) outcome and GOS 1 (dead) 1 year and 10–15 years after trauma by NfL and GFAP. The OR is the ratio of the odds for the dependent variable of GOS 1–3 outcome or GOS 1 with a relative increase in initial NfL or GFAP in the CSF of 50%. a GOS 1–3 outcome 1 year after trauma. b GOS 1–3 outcome 10–15 years after trauma. c GOS 1 outcome 1 year after trauma. d GOS 1 10–15 years after trauma. *The analysis was unadjusted due to few events. GOS 1–3 outcome at 1 year is adjusted for age. GOS 1–3 outcome and GOS 1 10–15 years after trauma are adjusted for age and neurological diseases. The results for OR, p-value and area under the ROC curve (AUC) are based on original values and not stratified groups. Each patients maximum CSF concentration of NfL and GFAP was used in the calculation. CSF cerebral spinal fluid, GFAP glial fibrillary acidic protein, GOS Glasgow Outcome Scale, NfL neurofilament light
Receiver operating characteristic curve (ROC) for NfL and GFAP in the CSF for GOS 1–3 outcome and GOS 1 outcome. ROC illustrating the quality at which specific levels of NfL and GFAP in the CSF can separate GOS 1–3 from GOS 4–5 outcome and GOS 1 outcome from GOS 2–5 outcome. a GOS 1 outcome 1 year after trauma. b GOS 1–3 outcome 10–15 years after trauma. c GOS 1 outcome 1 year after trauma. d GOS 1 10–15 years after trauma. The marked cut-off value is calculated using the sum of best sensitivity and specificity for each biomarker. Each patients maximum CSF concentration of NfL and GFAP was used in the calculation. CSF cerebral spinal fluid, GFAP glial fibrillary acidic protein, GOS Glasgow Outcome Scale, NfL neurofilament light
Initial Maximum CSF Concentrations of NfL and GFAP and Outcome 10–15 Years After an sTBI: GOS 1–3 (Poor) Versus GOS 4–5 (Good) Outcome
Both NfL and GFAP correlated with 10–15-year GOS 1–5 (rs = − 0.34, p = 0.026, and rs = − 0.37, p = 0.013), but after adjustment for age and neurological diseases, the correlation was lost (rs = − 0.27, p = 0.088, and rs = − 0.24, p = 0.13). Concentrations of NfL and GFAP per GOS category at 10–15 years after trauma are shown in Fig. 3b. Patients with GOS 1–3 outcome 10–15 years after trauma had significantly higher concentrations of both NfL and GFAP than those with GOS 4–5 outcome. After adjustment for age and neurological diseases, patients with GOS 1–3 outcome had significantly higher concentrations of NfL and GFAP (Table 3). Patients with GOS 4–5 outcome had (adjusted fold change) 64% lower NfL and 76% lower GFAP concentrations than patients with GOS 1–3 outcome 10–15 years after trauma (Table 3). We found significantly higher levels of NfL on days 0–2 and of GFAP on days 11–18 in patients with GOS 1–3 outcome than in those with GOS 4–5 outcome (Fig. 4b). After adjustments for age and neurological diseases, a relative increase in NfL and GFAP of 50% significantly increased the odds of a GOS 1–3 outcome 10–15 years after trauma (OR 2.04, p = 0.035, and OR 1.60, p = 0.040, respectively; Fig. 5b). At levels ≥ 10.9 μg/L (J = 0.49), NfL separated patients with a GOS 1–3 outcome from those with a GOS 4–5 outcome with a sensitivity of 0.78 and a specificity 0.71, whereas at levels ≥ 100 μg/L (J = 0.59), GFAP separated patients with a GOS 1–3 outcome with a sensitivity of 0.78 and a specificity of 0.81 (Fig. 6b).
Initial Maximum CSF Concentrations of NfL and GFAP and Mortality at 1 Year and 10–15 Years After an sTBI: GOS 1 (Dead) Versus GOS 2–5 (Alive)
Patients who died (GOS 1) within 1 year after trauma had significantly higher levels of NfL and GFAP than survivors (GOS 2–5) before and after adjustments for age (Table 3). Patients with GOS 2–5 outcome had (adjusted fold change) 81% lower NfL concentrations and 91% lower GFAP concentrations than patients with GOS 1 outcome 1 year after trauma (Table 3). We found significantly higher levels of NfL on days 0–2, 3–4, 6–8, and 11–18 and of GFAP on days 0–2, 3–4, and 6–8 in patients with GOS 1 compared with those with GOS 2–5 outcome (Fig. 4c). The number of patients with GOS 1 within 1 year was too low to allow adjustment analysis. Unadjusted, a relative increase in NfL and GFAP concentrations of 50% significantly increased the OR for dying (GOS 1) (OR 4.04, p = 0.0049, and OR 2.30, p = 0.0021, respectively; Fig. 5c). An NfL concentration of ≥ 14.7μg/L (J = 0.54) was able to separate patients with GOS 1 within 1 year after trauma with a sensitivity of 0.78 and a specificity of 0.76. At a concentration of 545 μg/L (J = 0.67), GFAP separated patients with GOS 1 within 1 year with a sensitivity of 0.78 and a specificity of 0.89 (Fig. 6c). Further, patients with GOS 1 within 10–15 years after trauma had significantly higher concentrations of NfL and GFAP than those with GOS 2–5. However, after adjustment for age and neurological diseases, this difference was lost (Table 3). There were no significant concentration differences in NfL or GFAP between patients with GOS 1 or GOS 2–5 outcome 10–15 years after trauma per sample period (Fig. 4d). Finally, a relative increase in NfL or GFAP concentrations was not significantly associated with mortality within 10–15 years after trauma, neither before nor after adjustments for age and neurological diseases (Fig. 5d).
Discussion
This study demonstrated that concentrations of CSF NfL gradually increased during the first 2 weeks after an sTBI, whereas CSF GFAP peaked during the initial days. High CSF concentrations of both biomarkers were associated with a GOS 1–3 (poor) outcome at 1 year and 10–15 years after trauma and only GOS 1 (dead) at 1 year but not 10–15 years after trauma.
The gold standard for evaluating brain injury severity is the GCS and CT images. These clinical variables, combined with age and pupil reactivity, among others, are used in a prognostic model developed by the IMPACT investigators to estimate 6-month outcome after a moderate to severe TBI [22]. Adding biomarkers, such as NfL and GFAP in serum, in models similar to International Mission for Prognosis and Analysis of Clinical Trails in TBI (IMPACT) has revealed improved predictive properties [6, 23, 24]. It is clinically important to identify and study objective factors, such as biomarkers, as these may help us to better evaluate the initial brain damage and further provide an early indication of the prognosis after an sTBI. This may lead to better individualized treatment and help direct hospital resources.
The presence and concentration of blood-based biomarkers in patients with TBI is being increasingly investigated but less so in the CSF. Although CSF and serum concentrations of NfL and GFAP cannot be compared completely because of passage over an injured BBB, the influence of the glymphatic system, and blood clearance, among others, our results appear to agree with studies of NfL and GFAP in serum. Similar to the NfL concentration noted over time in our population, the trajectory profile of NfL after TBI has previously been shown to increase gradually over time in both serum and the CSF [23, 24]. One study included part of our cohort [6]. GFAP concentrations in serum have previously been described to peak within 16–48 h after a TBI [9, 23, 25]. Similarly, we found that GFAP in the CSF peaked during the initial days but later than 48 h after trauma. It is possible to speculate that the later peak in the CSF, as noted in the present study, may be explained by the restoration of the disrupted BBB within 24 h post injury, as shown by others [26, 27], and the continuous accumulation in the CSF due to injured and reactive astrocytes [2]. Further, other studies suggest that a TBI disrupts the brain clearance by a reduced and impaired glymphatic function [28, 29].This may also lead to an earlier decrease of concentrations in the blood.
To be able to investigate whether concentrations of NfL and GFAP in the CSF relate to outcome 1 year and 10–15 years after trauma, we considered different confounders. We considered age and pretrauma existing neurological diseases to be confounding factors. After adjustment for age, we found that both NfL and GFAP in the CSF relate to 1-year outcome. Al Nimer et al. [24] found that unadjusted CSF NfL related to 6–12-month outcome after trauma; however, after adjustments for clinical variables, such as age, GCS, pupil response, and CT, the relationship was lost. Several studies have previously found that NfL and GFAP in serum relate to 3-month, 6-month, and 1-year outcome [6, 9, 23, 24, 30, 31]; two of them included patients from the present cohort [6, 9]. In agreement with our results in the CSF, NfL and GFAP in serum have been shown by others to be able to discriminate between a poor and a good outcome [30, 31]. One study included patients from our cohort [6]. Further, we found NfL and GFAP to relate to outcome 10–15 years trauma, adjusted for age and neurological disease. To our knowledge, we are the first to study how initial concentrations of NfL and GFAP in the CSF relate to outcome as long as 10–15 years after trauma. Patients with TBI can have elevated concentrations of NfL and GFAP in serum for month to years compared with controls [32, 33]. Newcombe et al. [33] found that higher levels of NfL in patients with TBI was related to worsening in functional outcome (Glasgow Outcome Scale Extended) between 8 months and > 5 years after trauma. Further, NfL levels in the chronic phase after a TBI have been found to be associated with brain volume loss on magnetic resonance imaging years following TBI, whereas GFAP did not [32, 33]. In contrast to these studies, we studied the initial acute concentrations of NfL and GFAP in CSF and not serum. We have previously shown that GOS at 1 year correlates with GOS 10–15 years after trauma, although individual improvement and deteriorations were noted [15]. The continuous relation between the acute-phase concentrations of NfL and GFAP in CSF and outcome 10–15 years after trauma is possibly a reflection of the long-lasting effect the initial injury has on function. In contrast, subacute and chronic levels may indicate ongoing processes and an association with later deterioration [32, 33].
When unadjusted, our data showed that CSF concentrations of NfL and GFAP related to mortality 1 year after trauma. However, when adjusted for age and neurological disease, NfL and GFAP did not relate to mortality at 10–15 years after trauma. Previous studies, two including some patients from the present study [6, 9], showed that NfL and GFAP in serum related to mortality 1 year after trauma [6, 9, 31]. McMillan et al. [34] found that up to 13 years after trauma, patients with sTBI had higher mortality, but the cause of death itself was similar to that in control patients. This suggests that factors other than the initial brain injury may influence late deaths after a TBI and may thus explain part of our results.
Clinically, biomarkers in blood are preferable, being easier to access and less invasive. Today, intraparenchymal pressure probes are gaining in popularity in ICP monitoring, making biomarkers in blood even more important. Several investigators have previously studied the prognostic abilities of NfL and GFAP in blood, but few have done so in the CSF [35]. In this study, intraventricular catheters were used for ICP monitoring, enabling us to analyze biomarkers in the CSF and contributing to the knowledge of how these biomarkers relate to long-term outcome, with the advantage of being analyzed in close proximity to the actual pathophysiological processes (i.e., trauma) [36]. Further, in a previous study by Shahim et al. [6], we compared levels of NfL in serum and CSF in study participants who were part of this patient cohort. This investigation demonstrated a correlation between trajectory CSF and serum levels over 12 days, with CSF levels being 100-fold higher than those noted in serum [6]. Thus, because CSF catheters are more rarely used, it seems appropriate to analyze serum NfL with the novel, more precise techniques.
Advantages in proteomic by employing techniques such as mass spectrometry in which multiple proteins can be analyzed simultaneously will improve our knowledge regarding the heterogenic pathophysiological mechanism involved after a TBI. Studying these large data sets will help find more specified biomarkers for diagnosis, injury progression, prognosis, and possible therapy targets in the future [37]. Today, mass spectrometry-based proteomics studies highlight the inflammatory response and give new insight to pathophysiological pathways after a TBI [38,39,40]. Meanwhile, studies like ours will be part of the foundation of knowledge helping to implement current knowledge into clinical practice.
This study is limited by its single-center design and small sample set, restricting the interpretation of our results. Patients from the entire southwestern part of Sweden who have an sTBI are transferred and treated at the NICU at Sahlgrenska University Hospital. However, patients deteriorating at the primary hospital are not transferred to the NICU, producing an unavoidable selection bias. At the NICU, children were also treated and included in the study (n = 6). Statistical analyses were performed with and without the children. Without the pediatric population, the statistical relation between NfL and GFAP in CSF and the 1-year and 10–15-year outcome was enhanced. A possible explanation may be the almost inverted baseline temporal profiles of these biomarkers in healthy children [41, 42]. Further, the recovery process and the pathophysiological mechanisms after a TBI may also differ from that of an adult [43]. Studies of pediatric traumatic brain injury and the prediction of outcome with biomarkers are scares. Therefore, the results including the children are presented in the Supplementary Material together with descriptive data of the pediatric population.
CSF samples were only collected when the attending neurosurgeon regarded the ICP as reasonably stable. As a result, some samples could not be obtained, which is an eventual selection bias. Because the CSF was collected intermittently, with some missing samples, we chose to use the maximum concentrations of NfL and GFAP instead of a specific time to calculate the receiver operating characteristic curve. We chose not to exclude outliers from our data. However, we used log transformation and nonparametric statistics. Because of the lack of CSF, 46 patients were excluded from this study. These missing data may affect our generalizability. However, the population included in this study is similar to the original population with regard to age, sex, and 1-year outcome [15]. Three patients (6%) did not participate in the second assessment. However, they were still alive at the second follow-up and are thus included in the analysis of being dead or alive. We did not use imputation and, if it is associated with exposure or outcome, it may have affected our results and the generalizability of the study. As previously discussed, we adjusted for age as a confounding factor. We also adjusted for pretrauma neurological diseases as a confounding factor, although the statistical significance for this factor disappeared when we omitted the pediatric population. We did not adjust for specific lesion types, locations, and severities. The Marshall score is an approximate and does not replace individual and aggregated information on specific intracranial lesion types.
Assuming that the brain damage and the recovery process would be stable 10–15 years after trauma, the second assessment was performed. To minimize loss to the second follow-up, the GOS interviews were held by phone. Finally, both interviewers assessing GOS were blinded to NfL and GFAP concentrations, but at the second assessment, the interviewers were not blinded to the 1-year neurological evaluation. We used the five-graded version of the GOS because it has higher agreement between investigators than the eight-graded Glasgow Outcome Scale Extended [20].
Conclusions
This study demonstrates that high concentrations of NfL and GFAP in CSF relate to a poorer outcome 1 year and 10–15 years after an sTBI. These results are in agreement with studies performed in serum at 1 year. Further, we found trajectory CSF profiles of both biomarkers. Our findings further contribute to our understanding of how biomarkers relate to both outcome and the initial injury. To implement prognostic markers in clinical practice as biomarkers of both prognosis and injury severity, we need larger studies to test and validate these biomarkers in models and studies designed to better define injury severity.
References
Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–41.
Yang Z, Wang KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38(6):364–74.
Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–89.
Lépinoux-Chambaud C, Eyer J. Review on intermediate filaments of the nervous system and their pathological alterations. Histochem Cell Biol. 2013;140(1):13–22.
Povlishock JT. Pathobiology of traumatically induced axonal injury in animals and man. Ann Emerg Med. 1993;22(6):980–6.
Shahim P, Gren M, Liman V, Andreasson U, Norgren N, Tegner Y, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6(1):36791.
Ost M, Nylén K, Csajbok L, Blennow K, Rosengren L, Nellgård B. Apolipoprotein E polymorphism and gender difference in outcome after severe traumatic brain injury. Acta Anaesthesiol Scand. 2008;52(10):1364–9.
Ost M, Nylén K, Csajbok L, Ohrfelt AO, Tullberg M, Wikkelsö C, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67(9):1600–4.
Nylén K, Ost M, Csajbok LZ, Nilsson I, Blennow K, Nellgård B, et al. Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J Neurol Sci. 2006;240(1–2):85–91.
Nylén K, Ost M, Csajbok LZ, Nilsson I, Hall C, Blennow K, et al. Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir (Wien). 2008;150(3):221–7.
Olsson A, Csajbok L, Ost M, Höglund K, Nylén K, Rosengren L, et al. Marked increase of beta-amyloid(1–42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol. 2004;251(7):870–6.
Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4.
Starmark JE, Stålhammar D, Holmgren E. The Reaction Level Scale (RLS85) Manual and guidelines. Acta Neurochir (Wien). 1988;91(1–2):12–20.
Grände PO. The “Lund Concept” for the treatment of severe head trauma—physiological principles and clinical application. Intensive Care Med. 2006;32(10):1475–84.
Andersson E, Rackauskaite D, Svanborg E, Csajbók L, Öst M, Nellgård B. A prospective outcome study observing patients with severe traumatic brain injury over 10–15 years. Acta Anaesthesiol Scand. 2017;61(5):502–12.
Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987(1):25–31.
Rosengren LE, Wikkelsø C, Hagberg L. A sensitive ELISA for glial fibrillary acidic protein: application in CSF of adults. J Neurosci Methods. 1994;51(2):197–204.
Marshall LF, Marshall SB, Klauber MR, Clark MB, Eisenberg HM, Jane JA, et al. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75(Suppl):S14-20.
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–4.
Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–85.
Crouchman M, Rossiter L, Colaco T, Forsyth R. A practical outcome scale for paediatric head injury. Arch Dis Child. 2001;84(2):120–4.
Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8): e165.
Thelin E, Al Nimer F, Frostell A, Zetterberg H, Blennow K, Nyström H, et al. A serum protein biomarker panel improves outcome prediction in human traumatic brain injury. J Neurotrauma. 2019;36(20):2850–62.
Al Nimer F, Thelin E, Nyström H, Dring AM, Svenningsson A, Piehl F, et al. Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PLoS ONE. 2015;10(7): e0132177.
Thelin EP, Zeiler FA, Ercole A, Mondello S, Büki A, Bellander BM, et al. Serial sampling of serum protein biomarkers for monitoring human traumatic brain injury dynamics: a systematic review. Front Neurol. 2017;8:300.
Logsdon AF, Meabon JS, Cline MM, Bullock KM, Raskind MA, Peskind ER, et al. Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci Rep. 2018;8(1):11344.
Başkaya MK, Rao AM, Doğan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226(1):33–6.
Park JH, Bae YJ, Kim JS, Jung WS, Choi JW, Roh TH, et al. Glymphatic system evaluation using diffusion tensor imaging in patients with traumatic brain injury. Neuroradiology. 2023;65(3):551–7.
Al Masri M, Corell A, Michaëlsson I, Jakola AS, Skoglund T. The glymphatic system for neurosurgeons: a scoping review. Neurosurg Rev. 2024;47(1):61.
Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62(8):1303–10.
Raheja A, Sinha S, Samson N, Bhoi S, Subramanian A, Sharma P, et al. Serum biomarkers as predictors of long-term outcome in severe traumatic brain injury: analysis from a randomized placebo-controlled Phase II clinical trial. J Neurosurg. 2016;125(3):631–41.
Shahim P, Politis A, van der Merwe A, Moore B, Ekanayake V, Lippa SM, et al. Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology. 2020;95(6):e623–36.
Newcombe VF, Ashton NJ, Posti JP, Glocker B, Manktelow A, Chatfield DA, et al. Post-acute blood biomarkers and disease progression in traumatic brain injury. Brain. 2022;145(6):2064–76.
McMillan TM, Teasdale GM. Death rate is increased for at least 7 years after head injury: a prospective study. Brain. 2007;130(Pt 10):2520–7.
Peters AJ, Schnell E, Saugstad JA, Treggiari MM. Longitudinal course of traumatic brain injury biomarkers for the prediction of clinical outcomes: a review. J Neurotrauma. 2021;38(18):2490–501.
Agoston DV, Shutes-David A, Peskind ER. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017;31(9):1195–203.
Sowers JL, Wu P, Zhang K, DeWitt DS, Prough DS. Proteomic changes in traumatic brain injury: experimental approaches. Curr Opin Neurol. 2018;31(6):709–17.
Lindblad C, Pin E, Just D, Al Nimer F, Nilsson P, Bellander BM, et al. Fluid proteomics of CSF and serum reveal important neuroinflammatory proteins in blood-brain barrier disruption and outcome prediction following severe traumatic brain injury: a prospective, observational study. Crit Care. 2021;25(1):103.
Shultz SR, Shah AD, Huang C, Dill LK, Schittenhelm RB, Morganti-Kossmann MC, et al. Temporal proteomics of human cerebrospinal fluid after severe traumatic brain injury. J Neuroinflammation. 2022;19(1):291.
Frik J, Merl-Pham J, Plesnila N, Mattugini N, Kjell J, Kraska J, et al. Cross-talk between monocyte invasion and astrocyte proliferation regulates scarring in brain injury. EMBO Rep. 2018;19(5):19.
Geis T, Gutzeit S, Fouzas S, Ambrosch A, Benkert P, Kuhle J, et al. Serum neurofilament light chain (NfL) levels in children with and without neurologic diseases. Eur J Paediatr Neurol. 2023;45:9–13.
Stukas S, Cooper J, Higgins V, Holmes D, Adeli K, Wellington CL. Pediatric reference intervals for serum neurofilament light and glial fibrillary acidic protein using the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) cohort. Clin Chem Lab Med. 2023;62(4):698–705.
Araki T, Yokota H, Morita A. Pediatric traumatic brain injury: characteristic features, diagnosis, and management. Neurol Med Chir (Tokyo). 2017;57(2):82–93.
Acknowledgements
We are grateful to all the participants and their families. We further wish to thank Ingrid Eiving, RN, for her help during patient inclusion and follow-up and the Neurochemical Laboratory in Mölndal for biomarker analysis. We are grateful for the professional work and statistical expertise provided by Statistiska Konsultgruppen, Gothenburg, who performed all the statistical analyses. A selected part of these results has been published as an abstract and presented orally, in Swedish, at a conference of the Swedish Society of Anesthesiology and Intensive Care (SFAI) in 2021.
Funding
Open access funding provided by University of Gothenburg. Henrik Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C and #ADSF-21-831377-C), the Olav Thon Foundation (No award/grant number), the Erling-Persson Family Foundation (no award/grant number), Stiftelsen för Gamla Tjänarinnor (No award/grant number), Hjärnfonden, Sweden (#FO2019-0228), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), European Union Joint Program for Neurodegenerative Disorders (JPND2021-00694), and the UK Dementia Research Institute at University College London (no award/grant number). Kaj Blennow is supported by the Swedish Research Council (#2017-00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the National Institute of Health (NIH), USA, (grant #1R01AG068398-01), and the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495). Bengt Nellgård is supported by the Swedish state under the agreement between the Swedish government and the County Councils the ALF-agreement (#ALFGBG-721141), Stiftelsen för Gamla Tjänarinnor (no award/grant number), Gun and Bertil Stohnes foundation (No award/grant number), and Hjalmar Svensson Foundation (no award/grant number).
Author information
Authors and Affiliations
Contributions
EA, MÖ, HZ, KB, and BN contributed to study design and concept. EA, MÖ, HZ, KB, and BN acquired data. HZ and KB contributed laboratorial analysis of NfL and GFAP in CSF. HZ, KB, and BN obtained funding. EA, MÖ, KD, HZ, KB, and BN contributed to analysis and interpretation of data. EA and MÖ drafted the first version of the manuscript. All authors revised the manuscript for important intellectual content. BN supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
Henrik Zetterberg has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Kaj Blennow has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Pharmatrophix, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. All other authors have no conflicts of interest.
Clinical Trial Registration
ClinicalTrials.gov identifier NCT05138692.
Ethical Approval/Informed Consent
The study was performed in accordance with the provisions of the Helsinki Declaration. The University Hospital Medical Ethics Committee, Gothenburg, Sweden, approved the initial study protocol (S-161 00) as well as a supplementary application for the secondary assessment (438-15). Informed written consent was obtained from each patient or next of kin.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The wrong Supplementary file was originally published with this article; it has now been replaced with the correct file.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Andersson, E., Öst, M., Dalla, K. et al. Acute-Phase Neurofilament Light and Glial Fibrillary Acidic Proteins in Cerebrospinal Fluid Predict Long-Term Outcome After Severe Traumatic Brain Injury. Neurocrit Care (2024). https://doi.org/10.1007/s12028-024-01998-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12028-024-01998-0