Abstract
Background
Alterations in perfusion to the brain during the transition from mechanical ventilation (MV) to a spontaneous breathing trial (SBT) remain poorly understood. The aim of the study was to determine whether changes in cerebral cortex perfusion, oxygen delivery (DO2), and oxygen saturation (%StiO2) during the transition from MV to an SBT differ between patients who succeed or fail an SBT.
Methods
This was a single-center prospective observational study conducted in a 16-bed medical intensive care unit of the University Hospital Leuven, Belgium. Measurements were performed in 24 patients receiving MV immediately before and at the end of a 30-min SBT. Blood flow index (BFI), DO2, and %StiO2 in the prefrontal cortex, scalene, rectus abdominis, and thenar muscle were simultaneously assessed by near-infrared spectroscopy using the tracer indocyanine green dye. Cardiac output, arterial blood gases, and systemic oxygenation were also recorded.
Results
During the SBT, prefrontal cortex BFI and DO2 responses did not differ between SBT-failure and SBT-success groups (p > 0.05). However, prefrontal cortex %StiO2 decreased in six of eight patients (75%) in the SBT-failure group (median [interquartile range 25–75%]: MV = 57.2% [49.1–61.7] vs. SBT = 51.0% [41.5–62.5]) compared to 3 of 16 patients (19%) in the SBT-success group (median [interquartile range 25–75%]: MV = 65.0% [58.6–68.5] vs. SBT = 65.1% [59.5–71.1]), resulting in a significant differential %StiO2 response between groups (p = 0.031). Similarly, a significant differential response in thenar muscle %StiO2 (p = 0.018) was observed between groups. A receiver operating characteristic analysis identified a decrease in prefrontal cortex %StiO2 > 1.6% during the SBT as an optimal cutoff, with a sensitivity of 94% and a specificity of 75% to predict SBT failure and an area under the curve of 0.79 (95% CI: 0.55–1.00). Cardiac output, systemic oxygenation, scalene, and rectus abdominis BFI, DO2, and %StiO2 responses did not differ between groups (p > 0.05); however, during the SBT, a significant positive association in prefrontal cortex BFI and partial pressure of arterial carbon dioxide was observed only in the SBT-success group (SBT success: Spearman’s ρ = 0.728, p = 0.002 vs. SBT failure: ρ = 0.048, p = 0.934).
Conclusions
This study demonstrated a reduced differential response in prefrontal cortex %StiO2 in the SBT-failure group compared with the SBT-success group possibly due to the insufficient increase in prefrontal cortex perfusion in SBT-failure patients. A > 1.6% drop in prefrontal cortex %StiO2 during SBT was sensitive in predicting SBT failure. Further research is needed to validate these findings in a larger population and to evaluate whether cerebral cortex %StiO2 measurements by near-infrared spectroscopy can assist in the decision-making process on liberation from MV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the majority of patients receiving mechanical ventilation (MV), weaning can be accomplished during the first liberation attempt; however, this is not the case for 15–30% of MV patients [1, 2]. Reducing the length of time patients are on MV decreases the risk of ventilator-associated complications, including respiratory and locomotor muscle weakness, infections, and mortality [3,4,5,6,7]. On the contrary, failure rates between 10 and 30% have been described during early discontinuation from MV [8,9,10,11,12]. Reintubated patients have a higher incidence of hospital mortality, increased length of intensive care unit (ICU) and hospital stay, prolonged duration of MV, and an increased need for tracheostomy [13]. Studies to identify parameters to predict weaning outcomes have demonstrated that several parameters, and not only respiratory ones, should be taken into account [8].
Energy supply to the respiratory muscles plays a critical role during the weaning process [14]. Elegant studies in animal models have shown a redistribution of blood flow away from the brain during spontaneous breathing to maintain an adequate energy supply to the respiratory muscles [15, 16]. More recently, studies in humans demonstrated that patients who fail a spontaneous breathing trial (SBT) can exhibit a reduction in perfusion in their splanchnic area and peripheral nonworking muscles that may be redistributed to the respiratory muscles [17, 18]. Nevertheless, alterations in perfusion to the brain during the transition from MV to SBT are still poorly understood in weaning patients.
Accordingly, this study aimed to assess changes in cerebral cortex perfusion, oxygen delivery (DO2), and tissue (i.e., cerebral cortex) oxygen saturation (%StiO2) during the transition from MV to SBT to determine whether these changes differ between patients who fail and patients who succeed an SBT. To address the aim of the study, we used near-infrared spectroscopy (NIRS) in conjunction with the injections of indocyanine green dye (ICG) [18,19,20,21] and simultaneously assessed local hemodynamic and tissue %StiO2 responses of the cerebral cortex (prefrontal cortex area), respiratory muscles, and nonworking muscles in consecutive weaning patients during MV and SBT. We hypothesize that SBT-failure patients would exhibit reduced cerebral cortex perfusion during the transition from MV to SBT compared with SBT-success patients.
Methods
Ethics
All procedures were performed in accordance with the ethical standards of the institutional review board of Universitair Ziekenhuis/Katholieke University Leuven and with the 1964 Helsinki Declaration and its later amendments, which have been described in the published protocol [23]. Ethical approval was obtained from the responsible local ethical committee (Ethische Commissie Onderzoek UZ/KU Leuven protocol ID: S60516), and the study was registered with ClinicalTrials.gov (identifier NCT03240263). Written informed consent was obtained from all patients. On the day of measurement, oral consent was allowed (i.e., nodding or shaking the head) only for patients who were unable to sign written consent because of physical weakness. This procedure was always performed in the presence of a witness (i.e., a nurse or doctor). Written consent was obtained afterward as soon as possible.
Study Design and Participants
This single-center prospective observational study was conducted in a 16-bed medical ICU of the University Hospital Leuven, Belgium, in 24 weaning patients between January 2019 and March 2020. Unconscious patients and patients unable to comprehend the study information were excluded from the study [23]. The inclusion criterion was patients on MV for more than 48 h who underwent their first SBT. Exclusion criteria were as follows: patients with acute or past brain injury (given concerns for motor deficits and brain swelling because the latter could affect the NIRS signal), preexisting neuromuscular disease, spinal cord injury above T8, skeletal pathology, severe kyphoscoliosis, congenital deformities, and contractures (given concerns for severe impairments in chest wall movement). Furthermore, we excluded patients with liver cirrhosis and chronic renal failure or with allergic reactions to iodine (given concerns for contradictions to ICG injections); patients with edema, trauma, or hematoma skin lesions at the sites of NIRS measurements (could hinder the placement of NIRS sensor probes); and patients with poor general prognosis or anticipated fatal outcomes [23]. This study complies with the Strengthening the Reporting of Observational Studies in Epidemiology statement.
Protocol
Measurements were performed during two conditions on the day clinicians judged that the patients were ready to wean: (1) on MV, immediately preceding the planned SBT, for a period of 30 min and (2) during a 30-min SBT (Fig. 1). A readiness-to-wean assessment was performed according to a local protocol [23]. The SBT in all patients was performed with either low (≤ 8 cm H2O) or zero level pressure support ventilation and continuous positive airway pressure (≤ 5 cm H2O). The mode of MV that was used prior to the SBT was continuous positive airway pressure with pressure support for 22 patients and bilevel positive airway pressure for two patients.
The timing of the different measures during MV and SBT. Black arrows indicate data collected at specific time points during MV and SBT. Dotted arrows indicate data recorded continuously during MV and SBT. CO cardiac output, FiO2 inspiratory oxygen fraction, Hb hemoglobin, HR heart rate, MAP mean arterial pressure, MV mechanical ventilation, PaCO2 partial pressure of arterial carbon dioxide, PaO2 partial pressure of arterial oxygen, PEEP positive end-expiratory pressure, pH hydrogen ion concentration, PS pressure support, P0.1 occlusion pressure at 100 milliseconds as an indicator of neuromuscular activation index of the respiratory system, RR respiratory rate, SaO2 arterial oxygen saturation, SBT spontaneous breathing trial, SV stroke volume, VE minute ventilation, VT tidal volume, %StiO2 fractional oxygen saturation
Readiness-to-Wean Criteria
Readiness-to-wean criteria included the assessment of the following: (1) resolution of the acute phase of the disease for which the patient was intubated, (2) adequate oxygenation (partial pressure arterial oxygen/inspiratory oxygen fraction [PaO2/FIO2] of 150–200 (P/F Ratio) requiring positive end-expiratory pressure [PEEP] ≤ 8 cm H2O and FIO2 ≤ 0.5), (3) absence of fever (temperature < 38 °C), (4) hemodynamic stability (e.g., heart rate ≤ 140 bpm), (5) stable blood pressure and no or minimal vasopressors (dobutamine ≤ 5 μg/kg/minute, norepinephrine ≤ 0.1 µg/kg/minute), (6) absence of myocardial ischemia, (7) adequate hemoglobin (e.g., hemoglobin level > 7–8 g/dL), (8) adequate mentation, and (9) adequate cough [23].
Evaluation of SBT Outcomes
Criteria assessed for evaluating the success or failure of the SBT included all of the following: (1) adequate gas exchange (Peripheral capillary oxygen saturation [SpO2] ≥ 85–90%, PaO2 ≥ 55–60 mm Hg, pH ≥ 7.32, and increase in partial pressure of arterial carbon dioxide [PaCO2] ≤ 10 mm Hg), (2) adequate ventilatory pattern (respiratory rate ≤ 30–35/minute, change in respiratory rate during SBT < 50%), (3) hemodynamically stable (heart rate < 120–140 bpm, changes in heart rate during SBT < 20%, systolic blood pressure < 180–200 mm Hg and > 90 mm Hg, and change in blood pressure during SBT < 20%), and (4) subjective clinical signs (no changes in mental well-being and comfortable, no sweating, no paradoxical breathing). The decision to extubate the patient was made by the treating clinician, independent of the investigators and blinded to measurements performed.
Hemodynamic Status Assessment
To assess whether any local changes observed might reflect changes in global hemodynamics, we assessed stroke volume, heart rate, and cardiac output continuously by pulse contour analysis using a specific sensor (Pulsioflex Monitor, Pulsion Medical Systems SE) [24, 25]. The device has been validated against clinical gold standard methods, and the results suggest that the pulse contour analysis method provides clinically acceptable cardiac output trend assessment, particularly in hemodynamically stable patients [26,27,28,29]. The calculation of cardiac output was performed five times per minute (every 12 s). Cardiac output data were exported in document file format and stored on disk for off-line analysis. Cardiac output and heart rate data were averaged over 60 s and were aligned with respiratory and tissues hemodynamic and oxygen saturation responses.
Ventilator Settings and Respiratory Parameters
Monitoring of the patients on MV before, during, and following the SBT was facilitated by using an electronic recording platform (MetaVision, iMD-Soft, Needham, MA) [30]. This platform can store information on patient demographics, anthropometric and clinical characteristics, and blood gas values measured by arterial blood gas analysis completed by the clinical team. The platform is also connected with the mechanical ventilator; thus, it continuously records and stores ventilatory settings data, such as pressure support and PEEP, as well as respiratory parameters, such as respiratory rate, tidal volume, minute ventilation, and FIO2 [30]. P0.1, which is the occlusion pressure at 100 milliseconds, was measured as an indicator of the neuromuscular activation index of the respiratory system [31]. P0.1 was measured in awake patients on MV immediately before the start of the SBT and at the end of the 30-min SBT.
Tissue Blood Flow Index andDO2 Assessments by NIRS
Cerebral cortex and respiratory and peripheral muscle blood flow indices (BFIs) were measured by a methodology validated in humans and various clinical populations [19,20,21,22, 32] using NIRS (Hamamatsu Photonics KK) in combination with injections of the tracer ICG (NIRS-ICG) [23]. Each injection included a dose of ICG of 5 mg diluted in 1 mL of saline, followed by a rapid 10-mL flush of isotonic saline [23].
The sets of NIRS optodes (light transmitter and emitter) were transcutaneously positioned as follows: for the brain over the prefrontal cortex area at an adequate distance to avoid interference with the midline sinus, for the scalene muscles on the left or right posterior triangle of the neck, and for the upper rectus abdominis on upper right third of rectus abdominis below the costal cartilage. The fourth set of NIRS optodes was placed on the thenar eminence muscle to represent a nonworking muscle site, as previously described [23].
Path lengths of 24.0 cm for the cerebral cortex and 18.0 cm for both respiratory and thenar muscles were set up according to the manufacturer’s recommendations. The separation distance between the NIRS light transmitter and receiver probes was 40 mm, thus allowing a maximum NIRS penetration depth of 20 mm. ICG concentration curve data were exported by NIRS to a document file format and stored on disk for off-line analysis. ICG concentration curves were analyzed by using the Chart5 version 5.4.2 (ADInstruments) program. A low-pass filter with a cutoff frequency of 0.5 Hz and smoothing window width (by using the triangular Bartlett window function) of nine points was used to smooth the ICG curves for BFI calculation. NIRS data were sampled at 6 Hz.
Tissue %StiO2 Assessment by NIRS
Continuous measurements of tissue %StiO2 were also performed by NIRS. NIRS-derived %StiO2 is a real-time and rapidly responsive absolute index of local tissue %StiO2 that is calculated by the ratio of microvascular oxygenated hemoglobin concentration (HbO2) to total hemoglobin concentration (tHb) and is expressed in percentage [(HbO2/tHb) × 100] [32]. %StiO2 reflects the dynamic balance between local tissue DO2 and consumption and therefore the capacity of tissue to match oxygen supplies relative to its metabolic demands [33]. Because of high ICG tissue concentrations, the passage of the dye bolus through the tissue may interfere with hemoglobin signals; thus, %StiO2 data (sampled at 6 Hz) were averaged over 15 s immediately before the ICG injection and were aligned with hemodynamic and respiratory responses.
Calculations
Systemic arterial O2 (SaO2) content was calculated using the following formula: [(1.34 × hemoglobin level × SaO2) + (0.003 × PaO2)]. Systemic DO2 was calculated by multiplying cardiac output and SaO2content. All blood gases were measured immediately before and after the SBT by arterial blood gas analysis. Tissue DO2 was calculated by multiplying tissue BFI and SaO2 content and was presented in arbitrary units (nanomole/second × milliliterO2).
The primary outcome of the study was the difference in change in cerebral cortex BFI during the transition from MV to SBT between SBT-success and SBT-failure patients. Secondary outcomes include the differences in change in respiratory and thenar muscle BFI, change in cerebral cortex and respiratory and thenar muscle %StiO2, ventilator settings, and change in breathing pattern parameters, hemodynamic parameters, and blood gas parameters [23].
Statistical Analysis
Categorical values are presented as numbers and proportions and compared using the χ2 test. Continuous data are expressed as median and interquartile range. The normality of the data was examined by the Shapiro–Wilk test. There were no missing data in this study. Within-group (before and after SBT) and between-group (SBT success and SBT failure) comparisons were performed by paired t-tests or Wilcoxon signed-rank tests and unpaired t-tests or Mann–Whitney U-tests, respectively, according to the distribution of the data. A mixed-model analysis of variance was applied to examine whether groups (i.e., SBT success and SBT failure) responded differently during MV versus at the end of the SBT for all the aforementioned physiological variables.
Because PaCO2 serves as one of the fundamental regulators of cerebral blood flow, Spearman's rank correlation was employed to determine the strength and direction of this relationship between the SBT-success and SBT-failure groups. Furthermore, if any of the cerebral cortex NIRS variables revealed a differential response between the SBT-success and SBT-failure groups, further exploratory analyses would involve the determination of the optimal cutoff, sensitivity, and specificity with the use of a receiver operating characteristic (ROC) curve. Specifically, an ROC curve was used to examine the diagnostic accuracy of the prefrontal cerebral %StiO2 to discriminate SBT failure from SBT success [34]. The Youden index method (i.e., the maximum of vertical distance of the ROC curve from the point [x, y] on the diagonal line [chance line]) was used to determine the optimal cutoff value of the prefrontal cerebral %StiO2 [35]. Statistical significance was defined as p < 0.05 (two-sided). Data were analyzed using the GraphPad Prism software.
Sample Size Calculation
The initial sample size of the study was estimated to be 20 SBT-failure patients [23], which was based on an expected effect size (Cohen’s d) of 0.467. This effect size was calculated from the mean difference of cerebral cortex BFI (i.e., 6.70 nmol/second) and the corresponding pooled standard deviation (i.e., 14.0 nmol/second), from a previous study investigating interhemispheric differences in cerebral cortex BFI in critically ill patients [20]. However, because of the outbreak of the COVID-19 pandemic in Belgium in March 2020, research activities were put on hold. Because the medical ICU of University Hospital Leuven in Belgium hospitalized patients with COVID-19 during all waves of the pandemic and no guarantee could be provided on when research activities would resume, inclusion in the trial was stopped prematurely.
Results
Characteristics of Patients
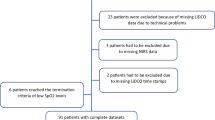
From January 2019 to March 2020, 287 consecutive patients from the medical ICU were screened for the study. Of those, 263 patients were excluded from the study because of poor general prognosis or anticipated fatal outcome (n = 99); chronic renal failure (n = 48); preexisting neuromuscular disease (n = 44); liver cirrhosis (n = 24); edema, trauma, or hematoma skin lesions at the sites of NIRS measurements (n = 22); acute or past brain injury (n = 8); spinal cord injury above T8 (n = 8); skeletal pathology that severely impaired chest wall movements (n = 7), and allergic reactions to iodine (n = 3). Therefore, the actual number of patients recruited for the study was 24 patients (i.e., n = 16 SBT-success patients and n = 8 SBT-failure patients). Demographics, anthropometrics, and clinical characteristics of all patients and the two subgroups separately (i.e., SBT success and SBT failure) are presented in Table 1. No differences in demographics and anthropometrics and clinical tools such as APACHE (Acute Physiology and Chronic Health Evaluation), SOFA (Sequential Organ Failure Assessment), GCS (Glasgow Coma Scale), and RASS (Richmond Agitation-Sedation Scale) scores were found between SBT-success and SBT-failure groups. Only patients in the SBT-failure group had the clinical diagnosis of heart failure (n = 2 of 8), and 4 of 8 (50%) patients had the clinical diagnosis of diabetes mellitus (vs. 13% in the SBT-success group). The SBT-failure group had a significantly longer duration of MV prior to the SBT compared with the SBT-success group (Table 1). All patients who failed the SBT failed at 30 min. The reasons recorded for the SBT-failure group were hypercapnia (n = 2 patients), hypoxemia (n = 2 patients), tachypnea (n = 2 patients), tachycardia (n = 1 patient), and changes in mental well-being and comfort (n = 1 patients). Extubation was successful for all patients in the SBT-success group.
Cardiovascular Parameters, Blood Gas Values, and Ventilatory Responses
During MV, cardiovascular parameters were similar between groups. Changes in cardiovascular responses when transitioning from MV to SBT were also similar for both groups (Table 2). During the transition from MV to SBT, percentage SaO2 was significantly reduced in the SBT-failure group. Differential responses of PaCO2 (p = 0.021), respiratory rate (p = 0.0006), and minute ventilation (p < 0.0001) were observed between SBT-failure and SBT-success patients (Table 2).
Local Tissue BFI and %StiO2 Responses
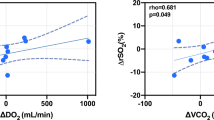
During the transition from MV to SBT, prefrontal cortex BFI significantly increased in the SBT-success group (p = 0.016) but not in the SBT-failure group (Table 3, Fig. 2a). We could not confirm a significant differential response in prefrontal cortex BFI between groups (Table 3). Similarly, prefrontal DO2 only significantly increased in the SBT-success group (p = 0.009; Table 3). In the SBT-success group, a strong and positive relationship was found between changes from MV to SBT in PaCO2 and prefrontal cortex BFI (ρ = 0.728, p = 0.002; Fig. 3) that was not observed in the SBT-failure group (ρ = 0.048, p = 0.934; Fig. 3). During the transition from MV to SBT, prefrontal cortex %StiO2 decreased in six of eight patients (75%) in the SBT-failure group, compared to 3 of 16 patients (19%) in the SBT-success group (p = 0.007), resulting in a significant differential %StiO2 response between groups (p = 0.031; Table 3, Fig. 2b). No differential responses for BFI, DO2, and %StiO2 were observed for scalene and abdominal muscles during the transition from MV to SBT between groups (Table 3). However, thenar muscle %StiO2 significantly decreased in the SBT-failure group, resulting in a differential response between groups (p = 0.018) (Table 3).
a Individual values of the prefrontal cortex blood flow index (BFI) during mechanical ventilation (MV) and spontaneous breathing trial (SBT) between SBT-success group (gray circles) and SBT-failure group (open circles). The reasons recorded for SBT failure were hypercapnia (patients #5 and #6), hypoxemia (patients #3 and #7), tachypnea (patients #1 and #8), tachycardia (patients #4), and changes in mental well-being and comfort (patients #2). b Individual values of the prefrontal cortex fractional oxygen saturation (%StiO2) during MV and SBT between SBT-success group (gray circles) and SBT failure group (open circles). The reasons recorded for SBT failure were hypercapnia (patients #3 and #6), hypoxemia (patients #2 and #7), tachypnea (patients #4 and #5), tachycardia (patients #1), and changes in mental well-being and comfort (patients #8)
Left panel: Scatter plot, Spearman’s rank correlation coefficients, and significance levels between changes from mechanical ventilation (MV) to spontaneous breathing trial (SBT) in cerebral cortex blood flow index (BFI) and partial pressure of arterial carbon dioxide (PaCO2) for the SBT-success group (gray circles) and SBT failure group (open circles). The reasons recorded for SBT failure were hypercapnia (patients #6 and #7), hypoxemia (patients #3 and #4), tachypnea (patients #2 and #5), tachycardia (patients #1), and changes in mental well-being and comfort (patients #8).
ROC Curve Analysis
A ROC curve showed that the optimal threshold of prefrontal cerebral cortex %StiO2 to predict SBT failure was > 1.6%, exhibiting 94% sensitivity, 75% specificity, and an area under the curve of 0.79 (95% confidence interval: 0.55–1.00; p = 0.024) (Fig. 4).
Receiver operating characteristic (ROC) curve for the cutoff point of the prefrontal cerebral oxygen saturation (%StiO2). The diagonal line represents a test that is expected a priori to have no discriminatory value. The Youden index method (i.e., the maximum of vertical distance of the ROC curve from the point [x, y] on the diagonal line [chance line] indicated by a black arrow) was used to determine the optimal cutoff value (red point) of the prefrontal cerebral %StiO2 (i.e., − 1.6%)
Discussion
To our knowledge, this is the first study that simultaneously and noninvasively assessed cerebral cortex and respiratory muscle perfusion, DO2, and %StiO2 responses on MV during an SBT. In contrast to our hypothesis, there was no differential response in perfusion and DO2 of the prefrontal cortex while transitioning from SBT between patients with SBT failure and patients with SBT success (Table 3). However, our findings did show a differential response in prefrontal cortex %StiO2 in the SBT-failure group compared with the SBT-success group (Table 3). The ROC curve indicated high sensitivity (i.e., 94%) and specificity (i.e., 75%) for a decrease of 1.6% in prefrontal cerebral cortex %StiO2 for the prediction of SBT failure (Fig. 4). The latter results suggest that prefrontal cortex %StiO2, in addition to the well-established broader physiological, respiratory, and psychological criteria, might be a useful tool to help predict SBT failure [36, 37].
NIRS-derived %StiO2 is the ratio of microvascular HbO2 to total hemoglobin (tHb), reflecting the balance between tissue DO2 and O2 consumption [32]. We postulate that the differential cerebral cortex %StiO2 response we found between SBT-success and SBT-failure groups may result from an insufficient increase in cerebral cortex perfusion in the latter group, necessary to meet cerebral cortex O2 requirements. Because NIRS mostly reflects venous %StiO2 [38] and therefore tissue oxygen extraction, the decrease in cerebral cortex %StiO2 values might indicate insufficient delivery to the cerebral cortex. Indeed, we observed a significant increase in prefrontal cortex perfusion and cerebral cortex DO2 in the SBT-success group, but not in the SBT-failure group (Table 3), during the transition from MV to SBT. This hypothesis is supported by the lack of association between PaCO2 and cerebral cortex BFI in the SBT-failure group, in contrast to the positive relationship we found in the SBT-success group (Fig. 3). Normal physiological response to hypercapnia includes a profound effect on cerebral blood flow because it causes marked dilation of cerebral arteries and arterioles and increased blood flow [39]. Therefore, the greater increase in PaCO2 in the SBT-failure group would be expected to act as a driver for a greater increase in cerebral BFI compared with the SBT-success group, which was not the case (Fig. 2).
The transition from positive pressure ventilation to SBT requires an increase in the DO2 to the respiratory muscles [40]. In both groups, there were significant increases in the perfusion and DO2 of scalene and abdominal muscles during the SBT to maintain adequate respiratory muscle oxygen supply (Table 3). Previous studies have reported that patients who fail an SBT may exhibit increased sympathetically mediated peripheral vasoconstriction, leading to restrictions in the oxygen availability of peripheral nonworking muscles in an attempt to support oxygen supply to the respiratory muscles [17, 18]. Our findings are consistent with others, demonstrating a significant reduction in thenar muscle %StiO2 in the SBT failure group (Table 3). In addition, we did not observe any differential responses in systemic oxygenation and hemodynamic response between SBT-success and SBT-failure groups (Table 2). Therefore, the differential response in the cerebral cortex and thenar muscle %StiO2 between groups (Table 3) cannot be explained by systemic oxygenation or hemodynamic changes.
The differential cerebral cortex %StiO2 response between SBT-success and SBT-failure groups could either be a marker profile or an epiphenomenon, or possibly this response could in part contribute to SBT failure. The prefrontal cortex receives input from multiple cortical regions and is involved in emotional, attention, motivational, executive function, and decision-making processes and may act as a relay station in the central nervous fatigue-related network, regulating central command through peripheral feedback [41]. The role of the prefrontal cortex functions in SBT failure was outside the scope of this study. Thus, studies to implement objective measures of neural respiratory drive by assessing esophageal pressure, transdiaphragmatic pressure, and respiratory muscles electromyography, together with cerebral %StiO2 measures, are necessary to confirm this hypothesis. Nevertheless, our data support previous observational studies showing that NIRS can be a valuable tool for the detection of cerebral ischemia [36]. Indeed, intraoperative and postoperative cerebral cortex deoxygenation, as determined by NIRS, can predict complications such as neurocognitive dysfunction, acute kidney failure, wound infection, and myocardial infarction following cardiac and noncardiac surgeries in adult and pediatric patients [42,43,44,45,46,47,48,49].
Our pilot study has important limitations. The study is limited by the single-center design and the small sample size. Therefore, the power to detect differences in baseline characteristics and certain outcomes, such as cerebral cortex BFI, may be limited. Although the study might be underpowered, we believe that the findings are novel and advance our understanding in this area of interest.
This study focused on the prefrontal cortex; thus, the “true” global brain response might not necessarily mirror the local responses investigated here in the prefrontal region of the forehead. Indeed, the pattern of blood flow responses could be different among brain regions, as indicated by studies measuring blood flow in the internal carotid and vertebral arteries in humans [50]. We chose to assess the perfusion and %StiO2 responses in scalene (in the absence of technology to assess the diaphragm) and rectus abdominis muscles because both muscles have been documented to play a critical role in SBT outcomes [51, 52]. We also took into account previously published data from our group showing appropriate scalene and rectus abdominis NIRS signal responsiveness under progressive respiratory efforts in patients with chronic lung diseases and critically ill populations [53, 54]. We did not assess the perfusion and %StiO2 responses in the sternocleidomastoid muscle to avoid potential NIRS signal artifacts caused by movements of the patient’s head. In addition, although supply could be maintained to scalene and rectus abdominis muscles, these data might not be applicable to other primary respiratory muscles, such as the diaphragm.
Another limitation of the study includes the nonstandardized setting of PEEP and pressure support ventilation during the SBT. Different levels of pressure support and PEEP may lead to variable responses in the work of breathing and respiratory muscle perfusion and %StiO2 responses in the two groups. Along these lines, we could also expect different behaviors of respiratory and cerebral cortex %StiO2 according to the specific SBT settings. This must be considered in future studies exploring SBT failure by NIRS. The SBT-failure group had a significantly longer duration of MV prior to the SBT compared with the SBT-success group. Furthermore, only patients in the SBT-failure group had the clinical diagnosis of heart failure (n = 2 of 8), and 4 of 8 patients (50%) had diabetes mellitus Table (1), with a potential mechanism of limited capability to augment systemic oxygen delivery. However, the central hemodynamic responses did not differ between SBT-failure and SBT-success groups (Table 2). Differences in the intubation cause, comorbidities, and days on MV between study groups must be considered as potential confounders in future studies exploring SBT failure by NIRS. In addition, the inclusion of patients with acute or chronic brain injuries should be considered in future studies exploring weaning failure by brain imaging techniques.
In this study, the assessment of primary physiological parameters at only two points (i.e., during MV and at the end of the SBT) did not allow us to explore the evolution of NIRS and other relevant physiological changes during the SBT to the timing of the SBT failure. This study design is similar to that of a recent study that used noninvasive methodologies, such as electrocardiography, echocardiography, and lung ultrasound evaluation before and after an SBT, to describe whether weaning-induced pulmonary edema occurred in patients with or without diaphragm dysfunction [55]. Finally, this study did not examine the reproducibility of the BFI method for measuring cerebral blood flow to avoid exposing the patients to unnecessary ICG injections to address issues beyond the primary scope of this study. However, previous data revealed that the coefficient of variation of the prefrontal cerebral cortex BFI measured by the NIRS-ICG technique during repeated measures (reproducibility was evaluated during six ICG injections at 5-min intervals in 14 critically ill patients) was low (i.e., median coefficient of variation: 10%, first and third interquartile range: 4.9–18.5%) [22].
Conclusions
To conclude, during the transition from MV to SBT, we identified a differential response in prefrontal cortex %StiO2 between SBT-failure and SBT-success patients, possibly due to the insufficient increase in prefrontal cortex perfusion in the SBT-failure group. During the SBT, a drop in prefrontal cerebral cortex %StiO2 > 1.6% was found to have the best association with SBT failure, with 94% sensitivity. This study is hypothesis generating, and a large multicenter study is now needed to confirm these findings to validate the role of NIRS-derived cerebral cortex %StiO2 responses in supporting decisions about the timing of liberation from MV.
References
Béduneau G, Pham T, Schortgen F, et al. Epidemiology of weaning outcome according to a new definition. The wind study. Am J Respir Crit Care Med. 2017;195:772–83.
McConville J, Kress J. Weaning patients from the ventilator. N Engl J Med. 2013;368:1068–9.
Ouellette DR, Patel S, Girard TD, et al. Liberation from mechanical ventilation in critically ill adults: an official American College of Chest Physicians/American Thoracic Society clinical practice guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. 2017;151:166–80.
Dres M, Jung B, Molinari N, et al. Respective contribution of intensive care unit-acquired limb muscle and severe diaphragm weakness on weaning outcome and mortality: a post hoc analysis of two cohorts. Crit Care. 2019;23:370.
Piva S, Fagoni N, Latronico N. Intensive care unit-acquired weakness: unanswered questions and targets for future research. F1000Res. 2019;8:F1000.
Sklar MC, Dres M, Fan E, et al. Association of low baseline diaphragm muscle mass with prolonged mechanical ventilation and mortality among critically ill adults. JAMA Netw Open. 2020;3:e1921520.
Burns KEA, Rizvi L, Cook DJ, et al. Ventilator weaning and discontinuation practices for critically ill patients. JAMA. 2021;23:1173–84.
Boles JM, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56.
Baptistella AF, Sarmento FG, da Silva KR, et al. Predictive factors of weaning from mechanical ventilation and extubation outcome: a systematic review. J Crit Care. 2018;48:56–62.
Farghaly S, Hasan AA. Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care. 2017;30:37–43.
Savi A, Teixeira C, Silva JM, et al. Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care. 2012;27(221):e1-8.
Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med. 2013;188:220–30.
Rothaar RC, Epstein SK. Extubation failure: magnitude of the problem, impact on outcomes, and prevention. Curr Opin Crit Care. 2003;9:59–66.
Kemper M, Weissman C, Askanazi J, et al. Metabolic and respiratory changes during weaning from mechanical ventilation. Chest. 1987;92:979–83.
Hussain SNA, Roussos Ch. Distribution of respiratory muscle and organ blood flow during endotoxic shock in dogs. J Appl Physiol. 1985;59:1802–8.
Viires N, Sillye G, Aubier M, et al. Regional blood flow distribution in dogs during induced hypotension and low cardiac output: spontaneous breathing versus artificial ventilation. J Clin Invest. 1983;72:935–47.
Bocquillon N, Mathieu D, Neviere R, et al. Gastric mucosal pH and blood flow during weaning from mechanical ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1555–61.
Gruartmoner G, Mesquida J, Masip J, et al. Thenar oxygen saturation during weaning from mechanical ventilation: an observational study. Eur Respir J. 2014;43:213–20.
Kuebler WM, Sckell A, Habler O, et al. Noninvasive measurement of regional cerebral blood flow by near-infrared spectroscopy and indocyanine green. J Cereb Blood Flow Metab. 1998;18:445–56.
Terborg C, Bramer S, Harscher S, et al. Bedside assessment of cerebral perfusion reductions in patients with acute ischaemic stroke by near-infrared spectroscopy and indocyanine green. J Neurol Neurosurg Psychiatry. 2004;75:38–42.
Louvaris Z, Habazettl H, Wagner H, et al. Near-infrared spectroscopy using indocyanine green dye for minimally invasive measurement of respiratory and leg muscle blood flow in patients with COPD. J Appl Physiol. 2018;125:947–59.
Wagner BP, Gertsch S, Ammann RA, et al. Reproducibility of the blood flow index as noninvasive, bedside estimation of cerebral blood flow. Intensive Care Med. 2003;29:196–200.
Louvaris Z, Van Hollebeke M, Dhaenens A, et al. Cerebral cortex and respiratory muscles perfusion during spontaneous breathing attempts in ventilated patients and its relation to weaning outcomes: a protocol for a prospective observational study. BMJ Open. 2019;9:e031072.
Chamos C, Vele L, Hamilton M, et al. Less invasive methods of advanced hemodynamic monitoring: principles, devices, and their role in the perioperative hemodynamic optimization. Perioper Med (Lond). 2013;17:19.
Erlanger J, Hooker DR. An experimental study of blood pressure and of pulse-pressure in man. Johns Hopkins Hosp Rep. 1904;12:145–378.
Manecke GR, Auger W. Cardiac output determination from the arterial pressure wave: clinical testing of a novel algorithm that does not require calibration. J Cardiothorac Vasc Anesth. 2007;21:3–7.
Kusaka Y, Yoshitani K, Irie T, et al. Clinical comparison of an echocardiograph-derived versus pulse counter-derived cardiac output measurement in abdominal aortic aneurysm surgery. J Cardiothorac Vasc Anesth. 2012;26:223–6.
De Backer D, Marx G, Tan A, et al. Arterial pressure-based cardiac output monitoring: a multicenter validation of the third-generation software in septic patients. Intensive Care Med. 2011;37:233–40.
Salzwedel C, Puig J, Carstens A, et al. Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center, prospective, randomized study. Crit Care. 2013;17:R191.
Hoffman M, Van Hollebeke M, Clerckx B, et al. Can inspiratory muscle training improve weaning outcomes in difficult to wean patients? a protocol for a randomised controlled trial (IMweanT study). BMJ Open. 2018;8:e021091.
Kuhlen R, Mohnhaupt R, Slama K, et al. Validation and clinical application of a continuous P0.1 measurement using standard respiratory equipment. Technol Health Care. 1996;4:415–24.
McCormick PW, Stewart M, Lewis G, et al. Intracerebral penetration of infrared light. Technicalnote J Neurosurg. 1992;76:315–8.
Boushel R, Langberg H, Olesen J, et al. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports. 2001;11:213–22.
Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp J Intern Med. 2013;4:627–35.
Fluss R, Faraggi D, Reiser B. Estimation of Youden index and its associated cutoff point. Biom J. 2005;47:458–72.
Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103:i3-13.
Tobias JD. Cerebral oxygenation monitoring: near-infrared spectroscopy. Expert Rev Med Devices. 2006;3:235–43.
Vogiatzis I, Habazettl H, Louvaris Z, et al. A method for assessing heterogeneity of blood flow and metabolism in exercising normal human muscle by near-infrared spectroscopy. J Appl Physiol. 2015;118:783–93.
Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol. 2017;102:1356–71.
Vassilakopoulos T, Zakynthinos S, Roussos C. Respiratory muscles and weaning failure. Eur Respir J. 1996;9:2383–400.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202.
Verges S, Rupp T, Jubeau M, et al. Cerebral perturbations during exercise in hypoxia. Am J Physiol Regul Integr Comp Physiol. 2012;302:R903-16.
Fedorow C, Grocott HP. Cerebral monitoring to optimize outcomes after cardiac surgery. Curr Opin Anaesthesiol. 2010;23:89–94.
Nielsen HB. Systematic review of near-infrared spectroscopy determined cerebral oxygenation during non-cardiac surgery. Front Physiol. 2014;5:93.
Phelps HM, Mahle WT, Kim D, et al. Postoperative cerebral oxygenation in hypoplastic left heart syndrome after the Norwood procedure. Ann Thorac Surg. 2009;87:1490–4.
Tortoriello TA, Stayer SA, Mott AR, et al. A noninvasive estimation of mixed venous oxygen saturation using near-infrared spectroscopy by cerebral oximetry in pediatric cardiac surgery patients. Paediatr Anaesth. 2005;15:495–503.
Moritz S, Kasprzak P, Arlt M, et al. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology. 2007;107:563–9.
Tobias JD, Hoernschemeyer DG. Near-infrared spectroscopy identifies compartment syndrome in an infant. J Pediatr Orthop. 2007;27:311–3.
Megan F, Hunt BS, Katharine T, et al. The use of cerebral NIRS monitoring to identify acute brain injury in patients with VA-ECMO. J Intensive Care Med. 2021;36:1403–9.
Sato K, Sadamoto T. Different blood flow responses to dynamic exercise between internal carotid and vertebral arteries in women. J Appl Physiol. 2010;109:864–9.
Parthasarathy S, Jubran A, Laghi F, et al. Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol. 2007;103:140–7.
Schreiber AF, Bertoni M, Coiffard B, et al. Abdominal muscle use during spontaneous breathing and cough in mechanically ventilated patients: a bi-center ultrasound study. Chest. 2021;S0012–3692(21):01084–9.
Louvaris Z, Rodrigues A, Dacha S, et al. High-intensity exercise impairs extradiaphragmatic respiratory muscle perfusion in patients with COPD. J Appl Physiol. 2021;130:325–41.
Hollebeke MV, Poddighe D, Clerckx B, et al. High-intensity inspiratory muscle training improves extradiaphragmatic inspiratory muscle oxygenation in patients with weaning difficulties: a randomized controlled trial. Front Physiol. 2022;13:786575.
Dres M, Rozenberg E, Morawiec E, et al. Diaphragm dysfunction, lung aeration loss and weaning-induced pulmonary oedema in difficult-to-wean patients. Ann Intensive Care. 2021;11:99.
Acknowledgements
We thank Ms Emily Hume (Northumbria University, Newcastle, UK) and Mr. Neeraj Shah, MD (Guy’s and St Thomas’ National Health Service, (NHS) Foundation Trust, London, UK), for language editing of the manuscript.
Funding
Dr Zafeiris Louvaris received a postdoctoral fellowship of the Research Foundation—Flanders (FWO) (#12U5618N). Prof. Greet Hermans received an FWO Senior Clinical Researcher Fellowship (#180512 N). Prof. Greet Hermans and Prof. Daniel Langer received an FWO project grant (#G053721N).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and designed research. ZL, MVH, and DP performed experiments and analyzed and visualized the data. All authors interpreted the results of the experiments. ZL drafted the manuscript. All authors revised and edited the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval/Informed Consent
Ethics approval was obtained from the responsible local ethical committee (Ethische Commissie Onderzoek Universitair Ziekenhuis/Katholieke University Leuven protocol ID: S60516). Written informed consent was obtained from all patients.
Clinical Trial Registration
ClinicalTrials.gov identifier NCT03240263.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Louvaris, Z., Van Hollebeke, M., Poddighe, D. et al. Do Cerebral Cortex Perfusion, Oxygen Delivery, and Oxygen Saturation Responses Measured by Near-Infrared Spectroscopy Differ Between Patients Who Fail or Succeed in a Spontaneous Breathing Trial? A Prospective Observational Study. Neurocrit Care 38, 105–117 (2023). https://doi.org/10.1007/s12028-022-01641-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01641-w