Abstract
Background
Augmented renal clearance (ARC) is a phenomenon that has been demonstrated in many subsets of critically ill patients and is characterized by a creatinine clearance (CrCl) > 130 mL/min. Prior research has examined ARC prevalence in the presence of sepsis, traumatic brain injury, subarachnoid hemorrhage, and intracranial hemorrhage. However, to our knowledge, no studies have examined whether this phenomenon occurs in patients suffering from an acute ischemic stroke (AIS). The objective of this study was to evaluate whether patients experiencing an AIS exhibit ARC, identify potential contributing factors, and examine the precision of current renal clearance estimation methods in patients with AIS experiencing ARC.
Methods
This was a single-center prospective observational study conducted in adult patients admitted to a neurocritical intensive care unit (ICU) at a community hospital. Once consent was gained, patients with an admitting diagnosis of an AIS underwent a 24-h urine collection to assess measured CrCl. The primary end point assessed for ARC, defined as a measured CrCl > 130 mL/min. The secondary end point evaluated length of stay in the neurocritical ICU.
Results
Twenty-eight patients met enrollment criteria, and data was analyzed for 20 patients. ARC was present in 35% of enrolled patients. Mathematical estimations of renal function were inadequate in detecting ARC manifestation. Patients experiencing ARC were associated with nonsignificantly shorter ICU length of stay.
Conclusions
ARC appears to manifest in patients with AIS inconsistently. Patients experiencing ARC were associated with nonsignificantly shorter ICU length of stay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Previous studies of critically ill patients have observed a phenomenon of enhanced renal clearance termed augmented renal clearance (ARC). ARC is defined as a creatinine clearance (CrCl) > 130 mL/min. Unidentified augmentation of renal clearance puts patients at risk for therapeutic failure due to subtherapeutic dosing strategies of renally eliminated medications, such as antibiotics or anticonvulsants.
ARC has been identified in critically ill patients with sepsis, subarachnoid hemorrhage, intracranial hemorrhage, hemorrhagic stroke, traumatic brain injury, trauma, burns, and febrile neutropenia [1]. Furthermore, several studies have demonstrated that ARC occurs significantly more often in certain patient demographics. These demographics include younger, mechanically ventilated men who have experienced mild trauma or polytrauma and are less frequently treated with vasopressors [3,4,5,6,7,8,9,10]. Current knowledge on ARC pathophysiology remains limited; however, many theories have been postulated, including aggressive fluid resuscitation, use of vasopressors, enhanced cardiac output, increased inflammatory mediators, and neuroendocrine alterations [1, 11]. Data regarding ARC onset from time of injury, when or if it hits a peak, and the duration, which we encompass by the term ARC window, are also limited.
There are currently two published ARC scoring systems that have been developed. The first published weighted scoring system was constructed from adjusted odds ratios obtained from a prospective observational study in septic and trauma patients. The scoring system assigned an age < 50 years six points, admission posttrauma three points, and a modified Sequential Organ Failure Assessment (SOFA) score less than 4 one point. A weighted score > 7 was associated with a sensitivity of 100% and specificity of 71.4% for identification of ARC [9]. An additional scoring system, termed Augmented Renal Clearance in Trauma Intensive Care (ARCTIC), was developed from a retrospective cohort study performed in trauma patients. The ARCTIC scoring tool evaluated ARC risk in the trauma intensive care unit (ICU) patient population. The scoring system was a point-based system assessing the following criteria: patients 56 years or younger received four points, patients 56–75 years received three points, a serum creatinine level less than 0.7 mg/dL received three points, and male patients received two additional points. An ARCTIC score of 6 or higher represents an appropriate cutoff at which antimicrobial adjustments may be considered for ARC [12].
Cerebral infarction secondary to an acute ischemic stroke (AIS) often results in severe and permanent neurological deficits. Although substantial research on ARC has been performed, there are no data on the prevalence of ARC in AIS. Our study is a single-center prospective observational study conducted in adult patients admitted to a neurocritical ICU that aims to evaluate the presence of ARC in the population of patients with AIS.
Several studies have evaluated the accuracy of standard calculations of renal function in the setting of ARC. Creatinine clearance (CrCl) can be estimated using the Cockcroft-Gault (CrClCG), modified Cockcroft-Gault (CrClCGM), and Modification of Diet in Renal Disease (CrClMDRD), equations. CrClCG (mL/min) was calculated as follows: (140 − age) × weight × 0.85 (if female)/(serum creatinine × 72). CrClCGM (mL/min/1.73 m2) was calculated as follows: (140 − age) × weight × 0.85 (if female)/(serum creatinine × 72 × body surface area). CrClMDRD (mL/min/1.73 m2) was calculated as follows: 175 × serum creatinine−1.54 × age−0.203 × 0.742 (if female) × 1.212 (if African American). Previous studies examining the performance of these estimators in the presence of ARC demonstrated that these estimated CrCl equations systematically underestimate the actual measured CrCl, thus rendering these mathematical equations inaccurate in the setting of ARC. Our study undertook an analysis of the efficacy of these equations in estimating actual measured CrCl in the stroke patient population. As a final end point, our study investigated the relationship between ARC and patient length of stay (LOS) in the ICU [2, 3, 11, 13,14,15,16].
Methods
Study Design, Setting, and Patient Selection
The study was approved by the CHRISTUS Health Institutional Review Board. Patients admitted in the neurocritical ICU between November 2019 and June 2021 were assessed in this single-center prospective observational study. In an attempt to increase the likelihood of accurately collecting all urine volumes, the neurocritical ICU was chosen for its lower nurse to patient ratio in comparison with medical surgical floors. The neurocritical ICU was also used in an attempt to protect the limited resources available to conduct this study. By only including patients in the neurocritical ICU, there would be less chance of collecting urine for a patient who might have been initially thought to have AIS but was found to have a different stroke-like syndrome following additional testing and evaluation. Patients were included if they were 18 years or older, were in the neurocritical ICU with an admitting diagnosis of AIS, and had an expected LOS greater than 24 h. Initially, magnetic resonance imaging (MRI) confirmation of an AIS was an inclusion criterion required for study enrollment. The protocol was amended in January 2020 to remove MRI confirmation as an inclusion criterion, and MRI was instead tracked as a baseline characteristic after the investigators noted delayed times to MRI attainment. Conditions warranting patient exclusion were acute kidney injury, preexisting renal dysfunction (chronic kidney disease stage 3, 4, and 5), renal replacement therapy, body mass index less than 18, trauma, malignancy, pregnancy, being in a prison population, and use of sulfamethoxazole/trimethoprim. Trimethoprim has demonstrated the ability to increase serum creatinine levels and thus decrease calculated CrCl. This change does not affect the glomerular filtration rate and results in a calculated CrCl that is falsely low. It is thought that this reversible increase is secondary to inhibition of renal tubule secretion of trimethoprim [24]. Acute kidney injury was defined as an increase in the serum creatinine level greater than 0.3 mg/dL within 48 h, an increase in the serum creatinine level greater than 1.5 times baseline known or presumed to have occurred within the prior 7 days, or urine volume less than 0.5 mL/kg/hour for 6 h.
Measured urine creatinine concentration is unestablished as standard of care at this institution, so informed consent was obtained prior to starting 24-h urine collection. Informed consent was obtained from the patient or next of kin if the patient was unable to provide consent. Urine was collected using foley catheters, external urinary devices, urinals, and bedside commodes. On enrollment, the provider placed an order in the electronic health record for a 24-h measured CrCl urine test. The neurocritical ICU nursing staff was educated on proper urine collection techniques prior to enrollment of each participant. Throughout the 24-h collection period, the collection container was stored on ice or refrigerated. Patients were further excluded in the study if the urine collection volume after the 24-h period was less than 1 L.
Data Collection and Analysis
Data collection through electronic health record medical chart review was conducted to assess patient demographics. Additional parameters assessed were vital signs, surgical interventions, medications administered, stroke characteristics, stroke severity using the National Institutes of Health Stroke Scale (NIHSS), and pertinent laboratory values.
Determination of Urine Creatinine Concentrations and CrCls
After completing the 24-h urine collection, the collection container was delivered to the hospital laboratory, where it was processed with the VITROS 7600 Integrated System. Serum creatinine that was obtained during the urine collection period was used to calculate measured CrCl. The measured CrCl was calculated as follows: urine creatinine × urine volume × 1.73 × body surface area]/(serum creatinine × 1440).
The primary outcome assessed for ARC is defined as measured CrCl greater than 130 mL/min. Secondary outcomes included ICU LOS as a surrogate for therapeutic failure secondary to subtherapeutic concentrations of antiepileptics and antimicrobials and the relationship between measured CrCl and estimated CrCl methods (CrClCGM, CrClMDRD). Estimated CrCl based on a serum creatinine level drawn during urine collection was compared with measured CrCl within the ARC group. Estimated CrCl was calculated using the CrClCGM and CrClMDRD formulas. The CrClCGM equation standardized the traditional CrClCG equation to body surface area. The CrClCGM has been found to closely correlate to measured CrCl. The CrClMDRD equation estimated glomerular filtration rate and is used in patients with estimated glomerular filtration rate levels > 60 mL/min/1.73 m2. Note the presence of scaling factors for women and African American patients [2, 3, 11, 13,14,15,16].
Statistical Analysis
Because a prior analysis had not been performed on this patient population, we aimed to enroll 20 patients in the study based on similar studies that assessed renal function in different patient populations and based on feasibility [3,4,5,6,7,8]. Excel spreadsheets were used for data entry. Descriptive analysis used mean, median, standard deviation, and percentages to summarize baseline characteristics. A one-sample t-test was used to assess presence of ARC in the AIS patient population, and a one-sample z-test was used to further calculate the proportion of patients with AIS who presented with ARC. A two-sample paired t-test was used to compare measured CrCl with estimated CrCl within the ARC group. Finally, a one-sample t-test was used to calculate the relationship between ICU LOS and ARC. All statistical calculations were done using the R statistical software package and Excel spreadsheets. A p value less than 0.05 was considered statistically significant [17].
Results
Demographic Data
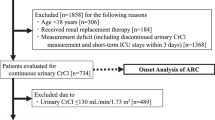
Between November 2019 and June 2021, 28 patients with AIS were enrolled in the study. Three patients were lost owing due to errors in urine collection methods. Five patients were excluded from the study for having urine volumes less than 1 L (Fig. 1). Data analysis was conducted on 20 patients. The demographics of the study population indicates a mean age of 59 years, with 60% male patients (Table 1). Mean admission CrClCG was 108 mL/min. Stroke was confirmed on MRI in 17 of the 20 patients, alteplase was administered in 75% of patients, contrast agents were administered for 85% of the population, and 60% of the patients underwent an endovascular thrombectomy procedure. Patients had a median NIHSS score of 12 during ICU admission and a median ARCTIC score of 5.
Primary and Secondary Outcomes
ARC was identified in 35% of the patients with AIS included in the data analysis. When we compared measured CrCl with CrClCGM, there was a statistically significant difference (164.3 ± 22.8 vs. 102.5 ± 31.7 mL/min/1.73 m2, respectively; p < 0.01). Measured CrCl in the ARC group also demonstrated a statistically significant difference when compared with CrClMDRD (164.3 ± 22.8 vs. 118.4 ± 23.6 mL/min/1.73 m2, respectively; p < 0.01). No other statistically significant differences were identified between the two groups. There was a nonsignificant trend toward lower ICU LOS in the ARC group (4 vs. 8 days; p = 0.06) (see Fig. 2).
Discussion
We believe this is the first study to assess ARC in patients with AIS. Because ARC impacts renally adjusted medications, such as antimicrobials and antiepileptics, at an enhanced rate, these patients may warrant higher doses and shorter frequencies to ensure adequate infection and seizure control. Currently, there are ARC-specific dosing recommendations for several medications, including piperacillin/tazobactam, ceftolozane/tazobactam, and levetiracetam.
We discussed both ARCTIC and ARC scores initially to provide a more thorough background on previously completed research on ARC. The ARC score assigns 3 of 10 total points if the patient experienced a trauma, leaving only age and the SOFA score as remaining factors. The ARC score would be irrelevant to the study at hand considering the nontrauma patient population. On the other hand, the ARCTIC score has more generalizable criteria and does not assign points on the basis of associated trauma. While both scoring systems have only been validated in trauma patients, the criteria for the ARCTIC score was generalizable enough to the expected non-trauma patient population that was worth exploring in this study.
ARC identification within the ischemic stroke population may be a result of an adaptive stress response. Abnormalities in the hypothalamus–pituitary–adrenal axis result in neuroendocrine alterations, with elevated levels of cortisol and catecholamines increasing metabolism and clearance. Additionally, natriuretic peptides (e.g., atrial natriuretic peptides and brain natriuretic peptides) with vasodilatory activities increase early after a stroke, with levels decreasing over time. These factors may contribute to the ARC captured in our seven patients [18].
Initially our protocol enrolled MRI-positive stroke patients to ensure our data captured kidney function in true ischemic strokes. However, time to MRI attainment largely varied, leading to a delay in the urine collection from the patient’s last seen normal time. After identifying the prolonged period to enrollment, the institutional review board protocol was amended to remove MRI as an inclusion criterion, and this characteristic was instead tracked as a patient baseline characteristic. In addition, on average patients were enrolled 58 h after their last seen normal time because informed consent could not be acquired if family members were unavailable at the bedside or because of the absence of a research enroller within the hospital. These factors may have led to missing a patient’s ARC window.
The study has several limitations to consider. Of our population, 85% of patients received contrast agents, thus potentially reducing kidney function by decreasing measured CrCl. Additionally, our hospital used a protocol to decrease indwelling catheter usage. The usage of bedside commodes, urinals, and other alternative urine collection methods in place of indwelling catheters over a 24-h collection period increased patient reluctance to enroll in the study. As a result, we may have had inconsistent and inaccurate urine collection methods. To correct for variable collection methods and the possibility of missed documentation, patients with a urine volume less than 1 L were excluded. The study generalizability may be limited due to a small sample size attributed to quick ICU discharge for stable patients, need for informed consent, and decreased census of stroke patients due to COVID-19.
By selecting patients on the neurocritical ICU, there may have been a selection bias for patients who were sicker. Patients with ARC did demonstrate a nonsignificant trend toward shorter ICU LOS, thus challenging our assumption for therapeutic failure secondary to subtherapeutic concentrations of antiepileptics and antimicrobials. This could be attributed to our small sample size or some correlation to the area affected by the ischemic stroke.
Despite these limitations, this remains a pioneer study in assessing ARC in patients with AIS. Although our results regarding the overall population mean CrCl being in the ARC range were nonsignificant, 35% of our patients with AIS included in this study did demonstrate ARC. Additionally, our data suggests traditional CrCl estimation methods are inadequate with identification of ARC. This study furthers our current understanding on ARC patient populations.
Throughout this study, we were unable to measure serial CrCl measurements to ascertain ARC onset, peak, and duration. Health systems with routinely used measured CrCl via urine collection could capture ARC more efficiently. Using an 8-h measured CrCl over a 24-h measured CrCl would increase data points and allow us to trend ARC. The 8-h measured CrCl would significantly reduce the duration of the urine collection period, decreasing reliance on nursing communication and the risk of variability in urine collection methods and increasing study feasibility.
Conclusions
Although the study results concerning the whole population of stroke patients were nonsignificant for ARC, we did identify a subset of AIS patient populations with ARC. Additionally, within the ARC group, the measured CrCl was significantly underestimated with the calculated CrCl equations. Patients with ARC were associated with shorter ICU LOS.
References
Cook AM, Hatton-Kolpek J. Augmented renal clearance. Pharmacotherapy. 2019;39(3):346–54.
May CC, Arora S, Parli S, et al. Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care. 2015;23(3):374–9.
Hoste EA, Damen J, Vanholder RC, et al. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant. 2005;20(4):747–53.
Udy A, Boots R, Senthuran S, et al. Augmented creatinine clearance in traumatic brain injury. Anest Analg. 2010;111:1505–10.
Udy AA, Baptista AP, Lim NL, et al. Augmented renal clearance in the ICU: results if a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med. 2014;42:520–7.
Udy AA, Jarrett P, Lassig-Smith M, et al. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma. 2017;34:137–44.
Conil JM, Georges B, Fourcade O, et al. Assessment of renal function in clinical practice at the bedside of burn patients. Br J Clin Pharmacol. 2007;63(5):583–94.
Morbitzer KA, Jordan JD, Dehne KA, et al. Enhanced renal clearance in patients with hemorrhagic stroke. Crit Care Med. 2019;47(6):800–8.
Barletta JF, Mangram AJ, Byrne M, et al. Identifying augmented renal clearance in trauma patients: validation of the augmented renal clearance in trauma intensive scoring system. J Trauma Acute Care Surg. 2016;82(4):665–71.
Loirat P, Rohan J, Baillet A, et al. Increased glomerular filtration rate in patients with major burns and its effects on the pharmacokinetics of tobramycin. N Engl J Med. 1978;299:915–9.
Mahmoud SH, Shen C. Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics. 2017;9(3):36.
Udy AA, Roberts JA, Shorr AF, et al. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care. 2013;17:R35.
National Kidney Foundation. 2021. CKD-EPI Creatinine Equation (2009).
Baptista JP, Udy AA, Sousa E, et al. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care. 2011;15:R139.
Adnan S, Ratnam S, Kumar S, et al. Select critically ill patients at risk of augmented renal clearance: experience in a Malaysian intensive care unit. Anaesth Intensive Care. 2014;42:715–22.
Baptista JP, Sousa E, Martins PJ, et al. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39:420–3.
R Core Team. R: a language and environment for statistical computing;2020. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
El Husseini N, Laskowitz DT. The role of neuroendocrine pathways in prognosis after stroke. Expert Rev Neuroth. 2014;14(2):217–32.
Grootaert V, Willems L, Debaveye Y, et al. Augmented renal clearance in the critically ill: how to assess kidney function. Nephrology. 2012;46:952–9.
Hirai K, Ishii H, Shimoshikiryo T, et al. Augmented renal clearance in patients with febrile neutropenia is associated with increased risk for subtherapeutic concentrations of vancomycin. Ther Drug Monit. 2016;38:706–10.
Carrie C, Petit L, d’Houdain N, et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 2018;51:443–9.
Ruiz S, Minville V, Asehnoune K, et al. Screening of patients with augmented renal clearance in ICU: taking into account the CKD-EPI equation, the age, and the cause of admission. Ann Intesive Care. 2015;5:49.
DeWaele JJ, Dumoulin A, Janssen A, Hoste EA. Epidemiology of augmented renal clearance in mixed ICU patients. Miner Anestesiol. 2015;81(10):1079–85.
Delanaye P, Mariat C, Cavalier E, et al. Trimethoprim, creatinine and creatinine-based equations. Nephron Clin Pract. 2011;119(3):c187–93.
Acknowledgements
The authors would like to thank the nurses on the CHRISTUS Mother Frances neurocritical care unit who were instrumental to the 24-hour creatinine clearance study. We would also like to thank the CHRISTUS Mother Frances laboratory department for absorbing the laboratory charges associated with the 24-hour creatinine clearance test. Lastly, we want to thank Dr. Jennifer Shupe for supporting our endeavor in assessing ARC and ordering the 24-hour creatinine clearance test.
Funding
Charges were absorbed by the CHRISTUS Mother Frances Hospital laboratory.
Author information
Authors and Affiliations
Contributions
GJ collected data and wrote the original draft. EH designed the study, collected data, and revised the manuscript. TC contributed to the study design and revised the manuscript. NS and RB analyzed the data and revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have nothing to disclose.
Ethical Approval/Informed Consent
We ensure that the work described has been conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. This study was approved by the CHRISTUS Institutional Review Board, and written informed consent was obtained from the patient or the legal medical decision-maker prior to initiation of any study procedures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law
About this article
Cite this article
John, G., Heffner, E., Carter, T. et al. Augmented Renal Clearance in Patients with Acute Ischemic Stroke: A Prospective Observational Study. Neurocrit Care 38, 35–40 (2023). https://doi.org/10.1007/s12028-022-01569-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01569-1