Abstract
Background
Continuous monitoring of cerebral oxygenation is one of the diagnostic tools used in patients with brain injury. Direct and invasive measurement of cerebral oxygenation with a partial brain oxygen pressure (PbtO2) probe is promising but invasive. Noninvasive assessment of regional transcranial oxygen saturation using near-infrared spectroscopy (NIRS) may be feasible. The aim of this study was to evaluate the interchangeability between PbtO2 and NIRS over time in patients with nontraumatic subarachnoid hemorrhage.
Methods
This retrospective study was performed in a neurocritical care unit. Study participants underwent hourly PbtO2 and NIRS measurements over 72 h. Temporal agreement between markers was described by their pointwise correlation. A secondary analysis assessed the structure of covariation between marker trajectories using a bivariate linear mixed model.
Results
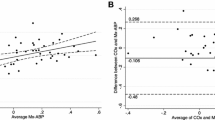
Fifty-one patients with subarachnoid hemorrhage were included. A total of 3362 simultaneous NIRS and PbtO2 measurements were obtained. The correlation at each measurement time ranged from − 0.25 to 0.25. The global correlation over time was − 0.026 (p = 0.130). The bivariate linear mixed model confirmed the lack of significant correlation between the PbtO2 and NIRS measurements at follow-up. NIRS was unable to detect PbtO2 values below 20 mm Hg (area under the receiver operating characteristic curve 0.539 [95% confidence interval 0.536–0.542]; p = 0.928), and percentage changes in NIRS were unable to detect a decrease in PbtO2 ≥ 10% (area under the receiver operating characteristic curve 0.615 [95% confidence interval 0.614–0.616]; p < 0.001).
Conclusions
PbtO2 and NIRS measurements were not correlated. There is no evidence that NIRS could be a substitute for PbtO2 monitoring in patients with nontraumatic subarachnoid hemorrhage.






Similar content being viewed by others
References
Peacock SH, Tomlinson AD. Multimodal neuromonitoring in neurocritical care. AACN Adv Crit Care. 2018;29(2):183–94.
Tran-Dinh A, Depret F, Vigué B. Pression tissulaire cérébrale en oxygène: pour quoi faire et pour qui? Ann Fr Anesth Réanim. 2012;31(6):e137–43.
Geeraerts T, Velly L, Abdennour L, Asehnoune K, Audibert G, Bouzat P, et al. Management of severe traumatic brain injury (first 24 hours). Anaesth Crit Care Pain Med. 2018;37(2):171–86.
Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(9):1189–209.
Soehle M, Jaeger M, Meixensberger J. Online assessment of brain tissue oxygen autoregulation in traumatic brain injury and subarachnoid hemorrhage. Neurol Res. 2003;25(4):411–7.
Scheufler K-M, Röhrborn H-J, Zentner J. Does tissue oxygen-tension reliably reflect cerebral oxygen delivery and consumption? Anesth Analg. 2002;95(4):1042–8.
Rosenthal G, Hemphill JC, Sorani M, Martin C, Morabito D, Obrist WD, et al. Brain tissue oxygen tension is more indicative of oxygen diffusion than oxygen delivery and metabolism in patients with traumatic brain injury*. Crit Care Med. 2008;36(6):1917–24.
Lin C-M, Lin M-C, Huang S-J, Chang C-K, Chao D-P, Lui T-N, et al. A prospective randomized study of brain tissue oxygen pressure-guided management in moderate and severe traumatic brain injury patients. BioMed Res Int. 2015;2015:1–8.
Okonkwo DO, Shutter LA, Moore C, Temkin NR, Puccio AM, Madden CJ, et al. Brain oxygen optimization in severe traumatic brain injury phase-II: a phase II randomized trial. Crit Care Med. 2017;45(11):1907–14.
Meixensberger J, Jaeger M, Vath A, Dings J, Kunze E, Roosen K. Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2003;74(6):760–4.
Veldeman M, Albanna W, Weiss M, Conzen C, Schmidt TP, Schulze-Steinen H, et al. Invasive neuromonitoring with an extended definition of delayed cerebral ischemia is associated with improved outcome after poor-grade subarachnoid hemorrhage. J Neurosurg. 2021;134(5):1527–34.
Shapiro S, Bowman R, Callahan J, Wolfla C. The fiberoptic intraparenchymal cerebral pressure monitor in 244 patients. Surg Neurol. 1996;45(3):278–81.
Kurth CD, Levy WJ, McCann J. Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia–ischemia in piglets. J Cerebral Blood Flow Metab. 2002;22(3):335–41.
Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11(4):274–81.
Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Danelli G, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. 2005;101(3):740–7.
Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104(1):51–8.
Roldán M, Kyriacou PA. Near-infrared spectroscopy (NIRS) in traumatic brain injury (TBI). Sensors. 2021;21(5):1586.
Rosenthal G, Furmanov A, Itshayek E, Shoshan Y, Singh V. Assessment of a noninvasive cerebral oxygenation monitor in patients with severe traumatic brain injury: clinical article. J Neurosurg. 2014;120(4):901–7.
Davies DJ, Clancy M, Dehghani H, Lucas SJE, Forcione M, Yakoub KM, et al. Cerebral oxygenation in traumatic brain injury: can a non-invasive frequency domain near-infrared spectroscopy device detect changes in brain tissue oxygen tension as well as the established invasive monitor? J Neurotrauma. 2019;36(7):1175–83.
Leal-Noval SR, Cayuela A, Arellano-Orden V, Marín-Caballos A, Padilla V, Ferrándiz-Millón C, et al. Invasive and noninvasive assessment of cerebral oxygenation in patients with severe traumatic brain injury. Intensive Care Med. 2010;36(8):1309–17.
Drake C. Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68(6):985–6.
Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9.
Thiébaut R, Jacqmin-Gadda H, Chêne G, Leport C, Commenges D. Bivariate linear mixed models using SAS proc MIXED. Comput Methods Programs Biomed. 2002;69(3):249–56.
Couch L, Roskosky M, Freedman BA, Shuler MS. Effect of skin pigmentation on near infrared spectroscopy. Am J Anal Chem. 2015;6(12):7.
Afshari A, Saager R, Zhou X, Ghassemi P, Lin J, Weininger S, et al. Skin pigmentation impact on cerebral oximetry: development and implementation of a phantom-based test method (conference presentation). SPIE; 2019.
Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103:i3–13.
Davie Sophie N, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation. Anesthesiology. 2012;116(4):834–40.
Büchner K, Meixensberger J, Dings J, Roosen K. Near-infrared spectroscopy - not useful to monitor cerebral oxygenation after severe brain injury. Zent Neurochir. 2000;61(02):69–73.
Naidech AM, Bendok BR, Ault ML, Bleck TP. Monitoring with the Somanetics INVOS 5100C after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2008;9(3):326–31.
Murkin JM. Cerebral oximetry. Anesthesiology. 2011;114(1):12–3.
Smith M, Elwell C. Near-Infrared spectroscopy: shedding light on the injured brain. Anesth Analg. 2009;108(4):1055–7.
Pennings FA, Schuurman PR, van den Munckhof P, Bouma GJ. Brain tissue oxygen pressure monitoring in awake patients during functional neurosurgery: the assessment of normal values. J Neurotrauma. 2008;25(10):1173–7.
Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10(1):122–8.
Acknowledgements
The authors thank Catherine Bigotto and Laure Estève for technical support and Musa Sesay for English-language editing.
Funding
This study was supported by institutional and departmental funds.
Author information
Authors and Affiliations
Contributions
HdC: recruited patients, collected the data, performed the statistical analysis, and wrote the manuscript. CP-L: analyzed the data and helped in writing the manuscript. ET: recruited patients, collected the data, and helped in writing the manuscript. DG: helped in the recruitment of patients, in data analysis, and in writing the manuscript. EV: helped in the recruitment of patients, in data analysis, and in writing the manuscript. MB: designed the study, helped in data analysis, and in writing the manuscript. All authors approved the final version.
Corresponding author
Ethics declarations
Conflicts of interest
MB received honoraria from Edwards Lifesciences and Pulsion Medical System as a lecturer. The other authors have no competing interests.
Ethical approval/informed consent
The study was approved by the Institutional Review Board of the French Society of Anesthesia and Critical Care (IRB 00010254-2018-032). According to French law, the patients and/or next of kin were informed about the inclusion of their anonymized data in the database, and none declined participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is related to the commentary available at https://link.springer.com/article/10.1007/s12028-022-01566-4.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Courson, H., Proust-Lima, C., Tuaz, E. et al. Relationship Between Brain Tissue Oxygen and Near-Infrared Spectroscopy in Patients with Nontraumatic Subarachnoid Hemorrhage. Neurocrit Care 37, 620–628 (2022). https://doi.org/10.1007/s12028-022-01563-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01563-7