Abstract
Background
Cerebral autoregulation plays an important role in safeguarding adequate cerebral perfusion and reducing the risk of secondary brain injury, which is highly important for patients in the neurological intensive care unit (neuro-ICU). Although the consensus white paper suggests that a minimum of 5 min of data are needed for assessing dynamic cerebral autoregulation with transfer function analysis (TFA), it remains unknown if the length of these data is valid for patients in the neuro-ICU, of whom are notably different than the general populations. We aimed to investigate the effect of data length using transcranial Doppler ultrasound combined with invasive blood pressure measurement for the assessment of dynamic cerebral autoregulation in patients in the neuro-ICU.
Methods
Twenty patients with various clinical conditions (severe acute encephalitis, ischemic stroke, subarachnoid hemorrhage, brain injury, cerebrovascular intervention operation, cerebral hemorrhage, intracranial space-occupying lesion, and toxic encephalopathy) were recruited for this study. Continuous invasive blood pressure, with a pressure catheter placed at the radial artery, and bilateral continuous cerebral blood flow velocity with transcranial Doppler ultrasound were simultaneously recorded for a length of 10 min for each patient. TFA was applied to derive phase shift, gain, and coherence function at all frequency bands from the first 2, 3, 4, 5, 6, 7, 8, 9, and 10 min of the 10-min recordings in each patient on both hemispheres. The variability in the autoregulatory parameters in each hemisphere was investigated by repeated measures analysis of variance.
Results
Forty-one recordings (82 hemispheres) were included in the study. According to the critical values of coherence provided by the Cerebral Autoregulation Research Network white paper, acceptable rates for the data were 100% with a length ≥ 7 min. The final analysis included 68 hemispheres. The effects of data length on trends in phase shift in the very low frequency (VLF) band (F1.801,120.669 = 6.321, P = 0.003), in the LF band (F1.274,85.343 = 4.290, P = 0.032), and in the HF band (F1.391,93.189 = 3.868, P = 0.039) were significant for 3–7 min, for 4–7 min, and for 5–8 min, respectively. Effects were also significant on the gain in the VLF band (F1.927,129.134 = 3.215, P = 0.045) for 2–8 min and on the coherence function in all frequency bands (VLF F2.846,190.671 = 90.247, P < 0.001, LF F2.515,168.492 = 55.770, P < 0.001, HF F2.411, 161.542 = 33.833, P < 0.001) for 2–10 min.
Conclusions
Considering the acceptable rates for the data and the variation in the TFA variables (phase shift and gain), we recommend recording data for a minimum length of 7 min for TFA in patients in the neuro-ICU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral autoregulation (CA) is the intrinsic ability of the brain to maintain adequate cerebral perfusion in the presence of blood pressure (BP) changes. CA represents the dynamic relationship between BP (stimulus or input) and cerebral blood flow (CBF) (response or output) [1]. It can be assessed by the correlation coefficient (Mx), autoregulation index (ARI), or transfer function analysis (TFA). Currently, TFA is the most widely applied approach in studies using spontaneous fluctuations of BP. However, there is still no standard interval for data collection for dynamic cerebral autoregulation (dCA) assessment with TFA. A consensus white paper for standardizing the use of TFA from the Cerebral Autoregulation Research Network (CARNet) suggests that recordings of spontaneous fluctuations of BP and CBF velocity (CBFV) for TFA should last for a minimum of 5 min to yield robust estimates of transfer function parameters [1]. Different data lengths have been used in past studies, most commonly 5 min [2, 3] or 10 min [4, 5]. A wide range of other data lengths have been reported [6,7,8,9]. Different data lengths may provide different results, which would then affect the accuracy of the measurement. A more accurate measurement is necessary for clinical application and research. Therefore, the main focus of this study was to explore the influence of different data lengths to provide a more robust assessment.
CA can be impaired in several disorders, such as brain injury [10], subarachnoid hemorrhage [11], acute cerebral hemorrhage [12], severe acute encephalitis [13], and ischemic stroke [14]. In neurological intensive care conditions, CA plays an important role in safeguarding adequate cerebral perfusion and reducing the risk of secondary brain injury. Moreover, the key role of BP control in the management of neurocritical care and the concept of optimal BP make the assessment of CA increasingly clinically important [15]. However, patients in the neurological intensive care unit (neuro-ICU) are notably different from those in the general population [15], and obtaining stable TFA parameters is relatively difficult under such conditions. For example, they are vulnerable to motion artifacts because of poor cooperation and clinical procedures. We therefore speculate that 5-min recordings may not be sufficient to assess CA, but long-term recordings may be difficult to obtain. The minimum data length collected for TFA has not been studied in patients in the neuro-ICU. It is necessary to determine the valid data length before using CA to guide clinical diagnosis and treatment.
Different equipment is used to measure CBFV and arterial BP (ABP) for TFA. CBFV recorded by transcranial Doppler ultrasound (TCD) is a reliable surrogate for CBF. ABP measured using noninvasive equipment, such as Finapres, has become the most common method. However, there is often not enough room for more bedside equipment in the neuro-ICU. In addition, the signal may fail to collect valid data because of poor peripheral circulation, such as the low temperature of the fingers. Most importantly, Finapres is not considered accurate enough for absolute continuous BP measurement, especially long-term measurement. Hence, using invasive BP measures and exporting data from the bedside monitor is an excellent solution to the inconveniences of noninvasive BP under neurological intensive care conditions. ABP measured by invasive placement of an intraarterial catheter in the radial artery is normally regarded as the gold standard [16] and should be the preferred method for dCA assessment in the intensive care unit [17]. Although there have been clinical and methodological studies [18] using invasive ABP, there has been no previous study evaluating the effect of data length on dCA assessment by means of TCD combined with an intraarterial catheter.
In summary, in this study, we attempted to use TCD combined with invasive ABP measurement to investigate the effect of data length on the assessment of dCA in patients in the neuro-ICU.
Methods
Participants
This prospective observational study was approved by the Ethics Committee of the Second Affiliated Hospital of Guangzhou University of Chinese Medicine (ZE2019-247–01). Patients admitted to the neuro-ICU at the Second Affiliated Hospital of Guangzhou University of Chinese Medicine from April 2020 to July 2020 were eligible for the study. All patients underwent the placement of a radial artery catheter to monitor ABP for clinical purposes. Patients with bilateral poor temporal windows found in TCD, middle cerebral artery peak flow velocity > 300 cm/s or < 40 cm/s, atrial fibrillation found in electrocardiography, or poor cooperation were excluded at the initial screening.
dCA Measurement
Measurements were performed in the neuro-ICU room by the same professional technician from 8:00 a.m. to 5:00 p.m. The room had a temperature-controlled environment of 22–24 °C. The patients were fed liquid nutrition through a small, lightweight, portable, and accurate enteral feeding pump (Flocare; Nutricia, Netherlands). Data collection was performed when patients were in a supine position. All patients received breathing assistance from a ventilator (Evita V300; Drager, Germany). CBFV was assessed using TCD (EMS-9 PB; Delica, China). Bilateral middle cerebral arteries at a depth of 45–60 mm were monitored through the temporal windows with 2-MHz probes attached to a head frame. BP was recorded simultaneously by a bedside monitor (BSM-6501C; Nihon Kohden, Japan) from an intravascular catheter inserted into the radial artery. Continuous CBFV and continuous ABP were recorded simultaneously for each patient for 10 min. Meanwhile, the end-tidal CO2 level was also monitored by the ventilator and maintained within a stable range (< 1 mm Hg). All analog signals were digitized and stored for editing and offline analysis. The data sampling frequency was 125 Hz.
Data Analysis
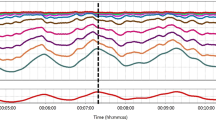
The data were processed using MATLAB (MathWorks, USA). The dynamic relationship between ABP and CBFV was analyzed by TFA, which was performed using the algorithm provided by CARNet with its default TFA parameters [1]. All recordings were required to be of high quality to ensure that the raw data were not removed or interpolated before the analysis (Fig. 1). A cross-correlation function between ABP and CBFV was used to align the data to eliminate possible time lags. The data were then downsampled to 1 Hz after passing through an antialiasing third-order Butterworth low-pass filter, which was applied with a cutoff frequency of 0.5 Hz. The averaged autospectra of ABP and CBFV, \(Sxx\left(f\right)\) and \(Syy\left(f\right)\), and cross-spectra of ABP and CBFV, \(Sxy\left(f\right)\), were estimated using Welch’s method. The periodograms of downsampled ABP and CBFV were averaged in the frequency domain with a 50% overlapping Hamming window of 90 s. The 3, 4, 5, 6, 7, 8, 9, and 10-min recordings consisted of 3, 4, 5, 7, 8, 9, 11, and 12 windows of data segments, respectively. However, the 2-min recording consisted of two windows with 66.7% data overlap. The transfer function,\(H\left(f\right)\), was then calculated as follows:
Then, the phase shift, \(\Phi \left(f\right)\), and the gain, \(\left|H\left(f\right)\right|\), were calculated as follows from \(H\left(f\right)\):
where \({H}_{R}\left(f\right)\) and \({H}_{I}\left(f\right)\) denote the real and imaginary parts of the transfer function \(H\left(f\right)\), respectively. Then, the magnitude-squared coherence function was calculated as follows:
The phase shift, gain, and coherence function from the first 2, 3, 4, 5, 6, 7, 8, 9, and 10 min of the 10-min recordings were then derived from TFA to evaluate dCA. A large phase shift in the low frequency (LF) band or low gain in the very low frequency (VLF) band indicates normal dCA. Because TFA is a linear model-based method, signals with low coherence between ABP and CBFV (< 0.51 in 3 windows, < 0.40 in 4 windows, < 0.34 in 5 windows, < 0.25 in 7 windows, < 0.22 in 8 windows, < 0.20 in 9 windows, < 0.17 in 11 windows, and < 0.15 in 12 windows, according to the critical values of coherence provided in the CARNet white paper) were excluded from further statistical analysis.
The ARI was implemented following Tiecks’ method [19]. The calculation is set out as follows:
where \(\mathrm{d}P\) is the normalized change in mean ABP \((\mathrm{MABP})\) from its control value (\(\mathrm{cABP})\), including the effect of the critical closing pressure (\(\mathrm{CCP})\).
where \({x}_{1}\) and \({x}_{2}\) are assumed to be equal to zero during the control phase, \(mV\) denotes the mean velocity and \(f\) represents the sampling frequency. The autoregulatory dynamic gain, \(K\), the time constant, \(T\), and the damping factor, \(D\), are the characteristic parameters of this mathematical model. Ten sets of \(K\), \(T\), and \(D\) values are used to correspond to different ARI values. The ARI value is determined by calculating the minimum root mean square error between the predicted and measured CBFV time series, according to the input ABP time series.
Statistical Analysis
Statistical data were analyzed using SPSS Statistics 17.0 (SPSS Inc, Chicago, IL). A P-value < 0.05 was considered statistically significant. Normality of the TFA-derived parameters was identified with the Shapiro–Wilk test. Normally distributed data are expressed as the mean ± standard deviation, and nonnormally distributed data are expressed as the median with interquartile range. The variabilities in the autoregulatory parameters in each hemisphere were investigated by repeated measures analysis of variance. In pairwise comparisons, the least significance difference post hoc test was used as a reference to identify where these differences occurred.
Results
Twenty patients (57.5 ± 13.8 years, 16 men) with 41 recordings (82 hemispheres) were included in the study. Admission diagnoses included severe acute encephalitis (n = 4), ischemic stroke (n = 3), subarachnoid hemorrhage (n = 3), brain injury (n = 3), cerebrovascular intervention operation (n = 3), cerebral hemorrhage (n = 2), intracranial space-occupying lesion (n = 1), and toxic encephalopathy (n = 1). Basic demographics and characteristics are listed in Table 1.
The mean ABP and mean CBFV are provided in Table 2. According to the critical values of coherence provided by the CARNet white paper, the acceptable rates for the data were 100% with a length ≥ 7 min; 14 hemispheres were excluded because of unacceptably low coherence of the 5-min recording. Thus, the final analysis contained 68 hemispheres. The dCA parameters (phase shift, gain, and coherence function) in all frequency bands are given in Table 3.
The variabilities in the dCA parameters in each hemisphere are shown in Fig. 2. The effect of data length on the trend in the phase shift in the VLF band was significant for 3 to 7 min (F1.801,120.669 = 6.321, P = 0.003) but not significant for 7 to 10 min (F1.637,109.651 = 0.230, P = 0.750). The effect of data length on the trend in the phase shift in the LF band was significant for 4 to 7 min (F1.274,85.343 = 4.290, P = 0.032) but was not significant for 7 to 10 min (F1.459,97.752 = 0.375, P = 0.622). The effect of data length on the trend in the phase shift in the high frequency (HF) band was significant for 5 to 8 min (F1.391,93.189 = 3.868, P = 0.039) but was not significant for 8 to 10 min (F1.262,84.571 = 1.000, P = 0.339). The effect of data length on the trend in the gain in the VLF band was significant for 2 to 8 min (F1.927,129.134 = 3.215, P = 0.045) but was not significant for 8 to 10 min (F1.283,85.990 = 0.280, P = 0.656).
The trends in the coherence function in all frequency bands were downward, and the effects of data length on the trends in the coherence function in all frequency bands were significant for 2 to 10 min (VLF F2.846,190.671 = 90.247, P < 0.001, LF F2.515, 168.492 = 55.770, P < 0.001, HF F2.411,161.542 = 33.833, P < 0.001).
The variabilities in ARI in each hemisphere are shown in Fig. 3. The effect of data length on the trend in ARI was not significant for 2 to 10 min (F3.366,225.502 = 1.064, P = 0.369). However, in pairwise comparisons, the result of the first 2 min was significantly decreased by 0.476 (0.054–0.899), compared with the result of the first 3 min (P = 0.028).
Discussion
The current study aimed to determine the valid data collection time for more robust dCA assessment in research or clinical applications. To achieve results with broad generalizability, a variety of severe neurological diseases were included in the admission diagnoses. Our results show that the dCA indices fluctuated significantly with data lengths of less than 7 min.
The magnitude of changes in the estimated marginal means of TFA parameters with shorter data lengths was relatively small. However, the fluctuation of quantitative results was obvious, especially in each individual patient. Starting with 2 min of data to approximately 7 min of data, we found that a short data length can cause significant variability in TFA variables. This finding is consistent with the recommendations of the white paper [1]. In addition, we found that a shorter data length could lead to a TFA value that changes from normal to abnormal (or vice versa). For example, taking 30° as the normal LF phase shift value, the qualitative result started to stabilize after 5 to 7 min. The variation resulting from shorter data length may be of little significance to clinical guidance. Perhaps the stability of the result might make dCA more clinically valuable.
Previous studies on data length showed that a 5-min recording was sufficient for dCA assessment. A 16-min recording study on the minimum length of data needed for the autoregulatory parameters to stabilize revealed that the phase shift in the LF band would be stable after 5 min [20]. A 10-min study comparing the dCA indices between 5 and 10-min data lengths indicated that phase shift was not significantly different between 5 and 10-min recordings, whereas gain and coherence were higher in the 5-min recording than in the 10-min recording [21]. A 5-min recording study comparing transfer functions estimated from different time lengths showed that transfer functions from the 3 and 2-min time series agreed very closely with the 5-min data in all frequency bands, whereas gain (but not phase shift) was overestimated in all frequency bands from the 1-min time series [22].
One explanation for the difference in the results between our study and previous studies may be the equipment. Four of the most common nonphysiological BP artifacts (saturation, square wave, reduced pulse pressure, and impulse) [23] and outliers [24] can influence the fluctuations of CA indices. Thus, invasive ABP monitoring was chosen for this study to reduce the effects of BP devices. To the best of our knowledge, this is the first study to use the preferred method for dCA assessment to investigate the effect of data length. However, there was a small but significant difference in the phase shift between noninvasive and invasive ABP for the assessment of dCA [17]. Invasive ABP monitoring may improve accuracy and is considered accurate enough for the measurement of absolute BP. In view of this, we expected that invasive BP might reduce the variabilities in the TFA parameters before our study began. However, the result was the opposite, and we attempted to find other explanations. Previous studies mainly analyzed healthy populations, except for Chi et al.’s study [21], which involved 37 patients with ischemic stroke, who were obviously different from patients in the neuro-ICU. The arterial tension of PaCO2, autonomic nervous system activity, body temperature, intracranial pressure, and intrathoracic pressure [25] can also affect the variability in CA estimates. dCA measurements were performed under less-controlled conditions in patients in the neuro-ICU. Furthermore, compared with healthy populations, the pathophysiological variations in patients in the neuro-ICU are more complex. Physiological variability or nonstationarity is likely to be the main reason for the poor reproducibility of dCA parameters [26]. Therefore, physiological conditions may require longer recordings to allow stationary physiological conditions. In addition, increased variability in BP leads to more robust estimates of autoregulation [27], whereas patients in the neuro-ICU need strict BP control. Finally, the nonlinear character of the underlying CA phenomena is potentially another important contributor to the high variabilities in the CA measures [20].
As the BP–CBFV relationship is assumed to be linear, it is worth paying attention to the coherence function, especially the acceptable coherence function. Similar to Chi et al. and Deegan et al.’s findings [21, 22], our results showed that coherence decreased as the data length increased. Most importantly, although coherence may decrease as the data length increases, the critical value of coherence can be reduced considerably as the number of windows increases. In other words, the phase shift and gain are valid as long as the coherence function is acceptable. In reference to our results, it is possible that phase shift and gain could not be calculated because of unacceptably low coherence if the data length was less than 7 min. However, all data were acceptable when they were collected for more than 7 min. It is unknown whether this results from the nature of TFA or is a physiological phenomenon.
Coincidentally, the phase shift and gain did not vary significantly after 7 min. Similar to Mahdi et al.’s study [20], the trend in phase shift was also downward in earlier minute intervals. Different from Chi et al.’s study [21], the trend in gain at the VLF band in our study was upward in earlier minutes, whereas their results showed that the gain in the 5-min recording was higher than that in the 10-min recording. Their results were explained through methodological issues such as the signal artifacts made by the Physiocal algorithm of the Finapres device. However, we did not have such problems because of the use of invasive ABP monitoring. Therefore, it is unclear whether these differences were due to the BP equipment used or the study population. In addition, a high value of phase shift and a low value of gain can indicate that CBFV is actively regulated against fluctuations in ABP, which leads to a good dCA. In contrast, a small phase shift and a large gain in TFA represent impaired dCA. In our study, the trends in the phase shift in the LF band and gain in the VLF band were consistent with each other.
As an additional analysis, we attempted to investigate the changes in ARI with different data lengths and found that ARI exhibited large variability before 3 min. This result is consistent with Mahdi et al.’s study [20], which suggested that the value of ARI could stabilize quickly and exhibit smaller fluctuations after 3 min. In addition, taking 5 as the normal ARI value, a shorter data length could still lead to an ARI value that changes from normal to abnormal (or vice versa). This finding was similar to our results regarding TFA parameters. Perhaps a longer data length is required to provide a more robust assessment.
This study has several limitations. First, intraarterial catheters are not preferred for CA assessment because local infection, arterial dissection, and pseudoaneurysm formation can occur. Second, the size of the sample in our study is small. Third, it is unclear whether a data length longer than 10 min would lead to a different result.
Conclusions
In conclusion, in this study, TCD monitoring and invasive ABP monitoring were used to investigate the effect of data length on the assessment of dCA with TFA. The trends in the CA measurements across different recording durations indicate that phase and gain vary significantly before 7 min. Combined with the result of the acceptable coherence rate, a recording length that is longer than 7 min may be more appropriate for dCA assessment of patients in the neuro-ICU.
References
Claassen JA, Meel-van DAA, Simpson DM, et al. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the international cerebral autoregulation research network. J Cereb Blood Flow Metab. 2016;36(4):665–80.
Eames PJ, Potter JF, Panerai RB. Assessment of cerebral autoregulation from ectopic heartbeats. Clin Sci (Lond). 2005;109(1):109–15.
Chi NF, Hu HH, Chan L, et al. Impaired cerebral autoregulation is associated with poststroke cognitive impairment. Ann Clin Transl Neurol. 2020;7(7):1092–102.
Castro P, Serrador J, Rocha I, et al. Heart failure patients have enhanced cerebral autoregulation response in acute ischemic stroke. J Thromb Thrombolysis. 2020;50(3):753–61.
Miller EC, Dos SK, Marshall RS, et al. Joint time-frequency analysis of dynamic cerebral autoregulation using generalized harmonic wavelets. Physiol Meas. 2020;41(2):24002.
Tian G, Ji Z, Huang K, et al. Dynamic cerebral autoregulation is an independent outcome predictor of acute ischemic stroke after endovascular therapy. Bmc Neurol. 2020;20(1):189.
Nakagawa K, Serrador JM, Larose SL, et al. Autoregulation in the posterior circulation is altered by the metabolic state of the visual cortex. Stroke. 2009;40(6):2062–7.
Liu X, Czosnyka M, Donnelly J, et al. Comparison of frequency and time domain methods of assessment of cerebral autoregulation in traumatic brain injury. J Cereb Blood Flow Metab. 2015;35(2):248–56.
Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol. 1998;85(3):1113–22.
Ding K, Tarumi T, Tomoto T, et al. Impaired cerebral blood flow regulation in chronic traumatic brain injury. Brain Res. 2020;1743:146924.
Otite F, Mink S, Tan CO, et al. Impaired cerebral autoregulation is associated with vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. Stroke. 2014;45(3):677–82.
Oeinck M, Neunhoeffer F, Buttler K, et al. Dynamic cerebral autoregulation in acute intracerebral hemorrhage. Stroke. 2013;44(10):2722–8.
Tang SC, Huang SJ, Chiu MJ, et al. Impaired cerebral autoregulation in a case of severe acute encephalitis. J Formos Med Assoc. 2007;106(2 Suppl):S7-12.
Aries MJ, Elting JW, De Keyser J, et al. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010;41(11):2697–704.
Claassen J, Thijssen D, Panerai RB, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021. https://doi.org/10.1152/physrev.00022.2020
Payne S. Cerebral autoregulation. Cham: Springer; 2016.
Petersen NH, Ortega-Gutierrez S, Reccius A, et al. Comparison of non-invasive and invasive arterial blood pressure measurement for assessment of dynamic cerebral autoregulation. Neurocrit Care. 2014;20(1):60–8.
Liu X, Czosnyka M, Donnelly J, et al. Assessment of cerebral autoregulation indices - a modelling perspective. Sci Rep. 2020;10(1):9600.
Tiecks FP, Lam AM, Aaslid R, et al. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26(6):1014–9.
Mahdi A, Nikolic D, Birch AA, et al. At what data length do cerebral autoregulation measures stabilise? Physiol Meas. 2017;38(7):1396–404.
Chi NF, Wang CY, Chan L, et al. Comparing different recording lengths of dynamic cerebral autoregulation: 5 versus 10 minutes. Biomed Res Int. 2018;2018:7803426.
Deegan BM, Serrador JM, Nakagawa K, et al. The effect of blood pressure calibrations and transcranial Doppler signal loss on transfer function estimates of cerebral autoregulation. Med Eng Phys. 2011;33(5):553–62.
Mahdi A, Rutter EM, Payne SJ. Effects of non-physiological blood pressure artefacts on cerebral autoregulation. Med Eng Phys. 2017;47:218–21.
R. KP. Outliers in process modeling and identification. IEEE T Contr Syst T. 2002; 10(1):55–63.
Panerai RB. Nonstationarity of dynamic cerebral autoregulation. Med Eng Phys. 2014;36(5):576–84.
Sanders ML, Elting J, Panerai RB, et al. Dynamic cerebral autoregulation reproducibility is affected by physiological variability. Front Physiol. 2019;10:865.
Liu J, Simpson DM, Allen R. High spontaneous fluctuation in arterial blood pressure improves the assessment of cerebral autoregulation. Physiol Meas. 2005;26(5):725–41.
Funding
None.
Author information
Authors and Affiliations
Contributions
Original draft preparation and methodology: Weijun Zhang. Conceptualization and investigation: Hongji Lu. Data analysis: Pandeng Zhang. Data collection: Xiuyun Mo. Statistical analysis: Aihua Ou. Article editing and validation: Jia Liu. Article review: Jingxin Zhong. The final manuscript was approved by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed Consent
Confirm adherence to ethical guidelines and indicate ethical approvals (institutional review board) and use of informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is related to the Invited Commentary available at https://doi.org/10.1007/s12028-021-01304-2.
Rights and permissions
About this article
Cite this article
Zhang, W., Lu, H., Zhang, P. et al. The Effect of Data Length on the Assessment of Dynamic Cerebral Autoregulation with Transfer Function Analysis in Neurological ICU Patients. Neurocrit Care 36, 21–29 (2022). https://doi.org/10.1007/s12028-021-01301-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01301-5