Abstract
Background
Red blood cell-induced cerebral inflammation and toxicity has been shown to be attenuated by induction of the heme-catalyzing enzyme, hemoxygenase-1 (HO-1), in animal models of subarachnoid hemorrhage (SAH). Although inflammatory mechanisms leading to secondary neuronal injury in SAH are becoming increasingly well understood, markers of cerebral inflammation have so far not been implemented in clinical prediction models of SAH.
Methods
In this biomarker observational study, HO-1 messenger ribonucleic acid (mRNA) expression levels were determined in cerebrospinal fluid (CSF) and blood of 66 patients with aneurysmal SAH on days 1, 7, and 14 after the SAH event. HO-1 mRNA expression was determined via real time polymerase chain reaction (PCR), and relative expression changes were quantified in comparison with expression levels in nonhemorrhagic control CSF. Subarachnoid blood burden, as well as presence of vasospasm and delayed cerebral ischemia (DCI), were recorded. Short and long-term clinical outcomes were assessed using the Modified Rankin Scale at discharge and 1 year after the SAH event.
Results
CSF HO-1 expression levels showed a significant increase over the 14-day observation period (p < 0.001, F = 22.53) and correlated with intracranial hematoma burden (ρ = 0.349, p = 0.025). In multivariate analyses, CSF HO-1 expression levels did not reach significance as independent predictors of outcome. Vasospasm on computed tomographic angiography was associated with lower CSF HO-1 expression levels on day 7 after SAH (n = 53, p = 0.010), whereas patients with DCI showed higher CSF HO-1 expression levels on day 14 after SAH (n = 21, p = 0.009).
Conclusions
HO-1 expression in CSF in patients with SAH follows a distinct temporal induction pattern and is dependent on intracranial hematoma burden. CSF HO-1 expression was unable to predict functional outcome. Associations of early low HO-1 expression with vasospasm and late elevated HO-1 expression with DCI may point to detrimental effects of late HO-1 induction, warranting the need for further investigation in a larger study population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) continues to be associated with high mortality and persisting disability rates [1], even more so in poor-grade SAH (Hunt & Hess or World Federation of Neurosurgical Societies [WFNS] grades 4 and 5) [2]. Even patients with favorable outcome [Modified Rankin Scale (mRS) < 3] frequently experience long-term cognitive impairment and decreased quality of life despite functional independence [3]. In addition to early brain injury induced by the acute hemorrhage, secondary complications, including vasospasm, hydrocephalus, and delayed cerebral ischemia (DCI), contribute substantially to long-term disability.
Over the past decades, it has become increasingly evident that, although the presence of vasospasm is associated with DCI and increased mortality and morbidity [4, 5], its successful treatment does not improve clinical outcome [6]. It has been postulated that angiographic vasospasm is merely one of multiple sequelae of red blood cell (RBC)-induced cerebral inflammation, whereas microthrombosis, impaired cerebral autoregulation, peripheral vasospasm, and cortical spreading depression represent other contributors to DCI [7,8,9], and that treatment of vasospasm alone is insufficient because it does not address the underlying inflammatory pathology [10]. Furthermore, oxidative stress has been associated with poor outcome and greater incidence of vasospasm after SAH [11, 12], whereas the attenuation of oxidative injury reduced neuronal apoptosis following SAH in a preclinical model [13].
Following aneurysm rupture, free RBC components in the subarachnoid space induce neuroinflammation, perturbations of the blood–brain barrier, and mediate direct cytotoxic effects on central nervous system cells, leading to edema [14,15,16]. The degradation of free heme into biliverdin, iron, and carbon monoxide (CO) is catalyzed by hemoxygenase enzymes (HO). The isoform hemoxygenase-1 (HO-1) is rapidly induced in models of traumatic brain injury, intracerebral and subarachnoid hemorrhage [17, 18]. Induction of microglial HO-1 has not only been shown to mediate the clearance of subarachnoid blood but also to attenuate neuronal injury and vasospasm in animal models after SAH [19,20,21]. HO-1 has also been demonstrated to exert protective effects against neuronal ischemia independently of intracranial hemorrhage (ICH) [22]. In patients with SAH, cerebrospinal fluid (CSF) HO-1 mRNA (messenger ribonucleic acid) expression levels were significantly elevated [19, 20] relative to controls without ICH. HO-1 expression levels also correlated with intracranial hematoma volume, underlining the importance of HO-1 activity for the clearance of subarachnoid blood [19]. In humans, the temporal pattern of CSF HO-1 expression after SAH has not been studied before. Because HO-1 is predominantly expressed in microglia after ICH [23, 24], the characterization of expression patterns could provide insight into neuroinflammatory processes.
Other markers of neuroinflammation have also been shown to be elevated even systemically. For instance, serum levels of the proinflammatory damage-associated molecular pattern molecule high-mobility group box-1 were elevated only in patients with SAH who developed vasospasm [25]. CSF interleukin-6 levels, although elevated in all patients with SAH, were significantly higher in patients who developed vasospasm [26, 27].
Despite growing understanding of the contribution of inflammation to secondary neuronal injury in SAH, markers of neuroinflammation have so far not been implemented in prediction and risk assessment in the clinical setting. Grading of SAH severity is predominantly based on neurological symptoms at disease onset and on the extent of subarachnoid blood burden on imaging [28,29,30].
The objective of this single-center observational study was to examine whether CSF and blood HO-1 mRNA expression levels of patients with SAH are indicators for intracranial hematoma burden and disease severity, and whether early measurement of HO-1 expression levels could predict short-term and long-term clinical outcomes, as sufficient HO-1 activity was associated with better functional outcome in murine models of SAH. Moreover, we sought to examine whether early measurement of HO-1 expression could identify patients at risk for secondary neurological deterioration after the initial bleeding event.
Methods
Study Design
A total of 66 patients with spontaneous SAH (19 men, 47 women, aged 58.03 ± 13.22 years, range 27–88 years, Table 1) admitted to either the intensive care units (ICUs) of the Department of Neurology and Neuroscience or the Department of Neurosurgery at the University of Freiburg (Germany) Medical Center between 2017 and 2020 were included in this study. The study protocol was approved by the Institutional Ethics Review Board of the University of Freiburg (Protocol no. 293/15). Informed consent from the patient, legal guardian, or by proxy was provided. The trial was registered with the German Clinical Trials Register (Trial-ID DRKS00008981; Universal Trial Number U1111-1172-6077).
The following criteria for inclusion were applied:
-
Age > 18 years.
-
Spontaneous SAH confirmed on computed tomography (CT) scan or via lumbar puncture/CSF xanthochromia.
-
Admission to the ICU and placement of an external ventricular drain (EVD) for therapeutic purposes, as well as first CSF and blood sample collection within 24 h after the SAH event.
-
Provision of informed consent from the patient, legal guardian, or by proxy.
The following criteria for exclusion were applied:
-
Age < 18 years.
-
Admission later than 24 h after symptom onset.
-
Current pregnancy.
-
Death within 24 h of ICU admission.
-
Evidence of septic aneurysm origin or evidence of ventriculitis or meningitis during the time period of sample collection.
-
Evidence of subdural or epidural hematoma on initial CT imaging.
Sample Collection and Analysis
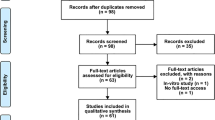
Cerebrospinal fluid and blood samples were obtained from patients with SAH on days 1, 7, and 14 after SAH symptom onset. Blood samples were obtained from either arterial or central venous catheters, whereas CSF samples were acquired under sterile conditions from EVDs placed for therapeutic purposes. If the patients’ external ventricular drain and/or arterial or venous catheters were removed because of clinical improvement or other reasons before day 7 or 14 after SAH symptom onset, sample collection was terminated (Fig. 1).
HO-1 mRNA expression levels from CSF and peripheral blood samples were determined as previously described.
Blood samples were stored at − 80 °C in RNA stabilizing reagent tubes (Tempus Blood RNA Tube, AB#4342792). RNA isolation from leukocytes was performed using the correspondent spin-column RNA isolation kit (Tempus Spin RNA Isolation Kit, AB#1710145). RNA content and purity were assessed photometrically (NanoDrop 2000 Spectrophotometer; Thermo Fisher Scientific Inc). RNA from CSF cells was isolated with TRIzolTM and concentrated by spin-column purification (RNeasy Micro Kit; Qiagen, Hilden, Germany). Reverse transcriptase polymerase chain reaction (PCR) technique (iScript cDNA (complementary deoxyribonucleic acid) Synthesis Kit, BioRad#1708890; PeqStar 96 Universal Gradient, PeqLab) was used to reversely transcribe RNA into cDNA. Semiquantification of cDNA was performed via real time PCR (sqPCR; StepOnePlus Real Time PCR-System, A&V Applied Biosystems) with nucleic acid stain (PowerUp SYBR Green Master Mix, AB#1708020) and specific primers for HO-1 and ribosomal protein L13a (Rpl13a), which served as intraindividual reference gene. CSF and blood samples of patients without ICH (patients receiving shunt surgery for normal pressure hydrocephalus) served as the interindividual reference population to calculate relative changes in mRNA expression levels. Expression levels of HO-1 mRNA were quantified using the 2−∆∆Ct method, in which ∆Ct (delta cycle threshold) = Ct (target gene HO-1) − Ct (reference gene Rpl13a) and ∆∆CT = ∆CT (study population) − ∆CT (reference population) [31].
Primer sequences were:
HO-1 forward | GTGATAGAAGAGGCCAAGACTG |
HO-1 reverse | GAATCTTGCACTTTGTTGCTGG |
Rpl13a forward | CGGACCGTGCGAGGTAT |
Rpl13a reverse | CACCATCCGCTTTTTCTTGTC |
Clinical Data Collection
As part of enrolment in the study protocol, and considering patients’ electronic charts and initial neuroimaging, the following clinical parameters were collected: demographic data (age and sex), SAH severity scores (Hunt & Hess grade, modified Fisher grade, WFNS grade), occurrence of sonographic or CT angiographic vasospasm during the course of ICU treatment, occurrence of DCI, and mRS score at admission, discharge, and 1 year after discharge.
Sonographic vasospasm [assessed with transcranial color-coded duplex sonography (TCCD)] was defined as mean blood flow velocity > 3 kHz (moderate)/ > 4 kHz (severe) or > 1 kHz increase compared to the examination on the previous day. TCCD results were corrected for age, vertebrobasilar system, heart rate, and hematocrit at the time of examination. Transcranial doppler sonography has been shown to have a high sensitivity (90%) to predict DCI [32]. DCI was defined as delayed onset of neurologic deterioration lasting > 24 h not explained by other causes, such as electrolyte disturbance or epileptic seizures or postictal deficits, or the presence of ischemic lesions on follow-up neuroimaging [33].
Outcomes were assessed using mRS [34] collected at hospital admission, discharge from hospital, and 1 year after SAH (assessment via telephone interview).
Radiographic total hematoma volume was assessed using OsiriX Lite software on the basis of the first CT scan after admission to the primary healthcare facility. First, cisternal hematoma volume (prepontine, interpeduncular, and ambient cisterns) was calculated from adjacent CT slices extending superiorly from the level of the caudal pons to the midbrain over a total vertical distance of 15 mm, taking slice thickness into consideration. Because cisternal hematoma volume alone often does not give an accurate representation of the total intracranial hematoma burden, ventricular and parenchymal hematoma volumes were calculated, if present, and added to the cisternal hematoma volume. In addition to this quantitative assessment, the semiquantitative Hijdra sum score [30] was determined for each patient’s initial CT scan.
Investigators performing clinical and radiographic ratings (mRS, hematoma volume) were blinded regarding PCR results and vice versa.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism software (version 8.4.3; GraphPad Software, San Diego, CA, www.graphpad.com).
According to previously published data [19], an a priori power analysis (Wilcoxon Mann–Whitney U-test, group comparison CSF HO-1 expression vs. mRS at discharge favorable/nonfavorable, effect size Cohen’s w 0.8; α = 0.05; power 95%, df 2) yielded a necessary sample size of 54 patients for biomarker discriminability.
Spearman nonparametrical correlation (ρ, p) was used to assess correlation between metrical data sets (total hematoma volume, HO-1 expression, mRS, Hunt & Hess, modified Fisher, and WFNS grades). The Mann–Whitney U-test was used for between-group comparisons (vasospasm yes/no, DCI yes/no, mortality yes/no, mRS 0–3 vs. 4–6, compared with HO-1 expression) of metrical data. Receiver operating characteristic (ROC) analyses were used to explore predictive power of HO-1 expression levels regarding functional outcome, vasospasm, and DCI. A mixed-effects analysis followed by Tukey’s multiple comparisons test was used to assess tendencies in repeated measures data (mRS, HO-1 expression). The relationship between CSF HO-1 expression and outcome [dichotomized into mRS 0–3 (favorable) and mRS 4–6 (nonfavorable)] was explored with multiple logistic regression, with age, hematoma volume, and WFNS (grade I–III vs. IV–V) as additional independent variables. Results are displayed as median and interquartile range. In all analyses, p values < 0.05 were considered statistically significant.
Results
Total Intracranial Hematoma Volume is an Indicator of Disease Severity
Total intracranial hematoma volume showed significant correlation with Hunt & Hess Grade (Fig. 2a, ρ = 0.570, p < 0.001), modified Fisher Score (ρ = 0.413, p = 0.002), and WFNS (ρ = 0.625, p < 0.001) Grade. Total hematoma volume also significantly correlated with mRS on admission (ρ = 0.600, p < 0.0001) and discharge (Fig. 2b, ρ = 0.469, p < 0.001).
Total hematoma volume is an indicator of disease severity. a Total hematoma volume significantly correlated with Hunt & Hess Grade, indicating disease severity (ρ = 0.570, p < .001, n = 52). b mRS at discharge, a measure of short-term functional outcome, also correlated with total hematoma volume (ρ = 0.469, p < .001, n = 52). mRS, Modified Rankin Scale
CSF HO-1 mRNA Expression Levels Increase After SAH
CSF HO-1 mRNA expression levels exhibited a significant increase during the 14-day time course of observation, indicating HO-1 induction that markedly outlasted the initial bleeding event (Fig. 3a, p < 0.0001, F1.692,60.92 = 22.53; day 1 vs. day 7 post SAH: p < 0.0001, n = 53, day 1 vs. day 14 post SAH: p < 0.001, n = 21, day 7 vs. day 14 post SAH: p = 0.051, n = 21).
CSF HO-1 expression is induced after the SAH event and is a function of disease severity. a CSF HO-1 mRNA expression levels significantly increased after the bleeding event (p < 0.0001, F (1.692,60.92) = 22.53). b and c, HO-1 mRNA expression levels on days 7 and 14 after SAH significantly correlated with total intracranial hematoma volume (day 7 post SAH: ρ = 0.349, p = 0.025, n = 41. Day 14 post SAH: ρ = 0.674, p = 0.002, n = 18). Column data are plotted as median with interquartile range and minimum to maximum, ***p < 0.001, ****p < 0.0001. CSF cerebrospinal fluid, HO-1 hemoxygenase-1, mRNA messenger ribonucleic acid, SAH subarachnoid hemorrhage
CSF HO-1 mRNA Expression Correlates with Total Hematoma Volume
CSF HO-1 mRNA expression levels on days 7 and 14, but not day 1, after SAH correlated significantly with total intracranial hematoma volume [day 1 post SAH: ρ = 0.056, p = 0.695, n = 51 (data not shown). Day 7 post SAH: ρ = 0.349, p = 0.025, n = 41, Fig. 3b. Day 14 post SAH: ρ = 0.674, p = 0.002, n = 18, Fig. 3c].
Systemic HO-1 mRNA Expression is Independent of Disease Severity
Subarachnoid hemorrhage leads to a rapid and substantial induction of HO-1 expression in the cerebrospinal fluid, with HO-1 expression levels rising over a period of 2 weeks after the bleeding event, the period of observation used in this study. The question arose whether HO-1 expression in blood leukocytes would mirror the distinct induction pattern observed in the CSF. However, HO-1 expression in blood leukocytes was induced to a far lesser extent than in the CSF [HO-1 expression day 7 post SAH in the CSF (fold induction vs. control): 339.3 (111.1–735.7), in blood leukocytes: 1.81 (1.16–13.14), median and interquartile range, Table 2]. There was also no significant change in blood HO-1 expression levels over the disease course (Fig. 4a, p = 0.289, F1.25,43.31 = 1.21), in contrast to the marked increase observed in the CSF.
Systemic HO-1 expression does not reflect cerebral HO-1 induction. a Blood leukocyte HO-1 mRNA expression levels did not exhibit a significant increase over the course of the disease (p = 0.289, F1.25, 43.31 = 1.21). b There was no correlation between blood and CSF HO-1 mRNA expression levels (day 7 post SAH: ρ = 0.144, p = 0.317, n = 50). Column data plotted as median with interquartile range and minimum to maximum. CSF cerebrospinal fluid, HO-1 hemoxygenase-1, mRNA messenger ribonucleic acid, SAH subarachnoid hemorrhage
Also, no correlation was found between CSF and blood HO-1 expression levels, determined at the same time points on days 7 after SAH (Fig. 4b), as well as days 1 and 14 (data not shown).
HO-1 Expression is Not Predictive of Functional Outcome
Over the course of ICU treatment and ensuing rehabilitation, patients showed significant overall clinical improvement as measured by the mRS [Fig. 5a, p < 0.0001, F1.354, 77.19 = 22.63; admission vs. discharge: p = 0.0002 (n = 66), admission vs. after 1 year: p < 0.0001 (n = 50), discharge vs. 1 year: p = 0.0013 (n = 50)].
CSF HO-1 expression levels according to short-term and long-term neurological outcome. a Over the course of ICU treatment and one year after the SAH event, patients showed significant clinical improvement measured by mRS (p < 0.0001, F1.354, 77.19 = 22.63, n = 50). b Patients with unfavorable short-term outcome showed significantly higher HO-1 expression levels on day 14 after SAH (n = 21, p = 0.014). c This effect could still be observed regarding long-term functional outcome (n = 16, p = 0.008). Data are plotted as mean + SEM (mRS) and median with interquartile range and minimum to maximum. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. CSF cerebrospinal fluid, HO-1 hemoxygenase-1, ICU intensive care unit, mRNA messenger ribonucleic acid, mRS Modified Rankin Scale, SAH subarachnoid hemorrhage, SEM standard error of the mean
The observational study design followed the natural clinical course of ICU treatment. Therefore, the endpoint of sample collection was reached once the EVD was removed because of clinical improvement (CSF samples day 1 after SAH: n = 65, day 7: n = 53, day 14: n = 21). This led to patients included in all 3 CSF sampling points representing a more severely affected subgroup of the total patient collective (mRS at discharge among patients with day 7 CSF samples: 3.45 ± 1.56, day 14: 4.28 ± 1.06, p = 0.035). 13.6% of the study population developed EVD-associated complications (ventriculitis: n = 5, intraparenchymal hematoma: n = 4).
HO-1 mRNA expression levels on days 1 and 7 after SAH did not differ between patient groups with favorable (mRS 0–3) or unfavorable (mRS 4–6) outcome, measured at discharge from hospital (short-term, Fig. 5b) and 1 year after the SAH event (long-term, Fig. 5c). However, higher day 14 CSF HO-1 expression levels were found in patients with unfavorable outcome at discharge from hospital and 1 year after the SAH event than in those with favorable outcome [Fig. 5b, c, mRS at discharge 0–3: 207.5 (28.05–531.7), n = 6, mRS at discharge 4–6: 830.9 (443.9–1792) (fold induction vs. control), n = 15, p = 0.014; mRS at 1 year 0–3: 213.4 (54.75–500.4), n = 9 vs. mRS at 1 year 4–6: 830.9 (652.5–1624) (fold induction vs. control), n = 7, p = 0.008]. Multiple logistic regression models for outcome (mRS at discharge favorable/nonfavorable) did not show a significant association between CSF HO-1 expression levels on days 7 or 14 and outcome (Table 3, Model 1: mRS discharge ~ intercept + CSF HO-1 Day 7 + WFNS grade IV–V yes/no + total hematoma volume + age, CSF HO-1 day 7 OR 1.001 [95% confidence interval {CI} 0.99–1.00], p = 0.28; area under the curve (AUC) 0.83 [95% CI 0.70–0.96], p = 0.0004; Model 2: mRS discharge ~ intercept + WFNS grade IV–V yes/no + total hematoma volume + age, AUC 0.84 83 [95% CI 0.74–0.95], p < 0.0001; Model 3: mRS discharge ~ intercept + CSF HO-1 day 14 + total hematoma volume + age, CSF HO-1 day 14 OR 0.99 [95% CI 0.97–0.99], p = 0.18; AUC 0.91 [95% CI 0.77–1.0], p = 0.009). It should be noted that the variables CSF HO-1 day 14 and WFNS could not both be included in one multiple logistic regression model because of multicollinearity (Spearman rank correlation p = 0.0008, r = 0.70). Furthermore, the number of patients still relying on an external ventricular drain 14 days after SAH was small (n = 21), limiting interpretability of multivariate analyses.
Early HO-1 Induction May Protect Against Development of Vasospasm, Whereas Late HO-1 Expression is Associated with Occurrence of DCI
When analyzing patient subgroups, patients that exhibited sonographic vasospasm at any point in time during hospital treatment showed a nonsignificant trend toward lower HO-1 mRNA expression levels on day 7 after SAH than those without sonographic vasospasm [Fig. 6a, HO-1 mRNA expression level 282.1 (89.15–571.5) vs. 634.8 (224.4–1326) (fold induction vs. control), n = 50, p = 0.059]. Regarding vasospasm detected on CT angiography, patients with manifest moderate or severe vasospasm on CT angiography had significantly lower CSF HO-1 expression levels on day 7 after SAH, compared with those who either received a CT angiography that did not show relevant vasospasm and those in which no CT angiography was performed [Fig. 6b, HO-1 expression level 119.5 (18.06–346.9) vs. 466.3 (199.7–1004) (fold induction vs. control), n = 53, p = 0.010]. ROC curve analysis showed an AUC of 0.73 with a 95% confidence interval of 0.57 to 0.89 (p = 0.01, Fig. 7a). At a sensitivity of 78.57% and specificity of 64.1%, predictive power was insufficient (positive predictive value (PPV): 56.41%, negative predictive value (NPV): 44.29%, HO-1 expression level cutoff > 301.5). There was no difference in HO-1 mRNA expression levels on day 14 after SAH in patients with or without vasospasm (sonography: n = 19, p = 0.875, CT angiography: n = 21, p = 0.287).
Early HO-1 expression may influence the development of vasospasm. a Patients who developed sonographic vasospasm showed a trend toward lower HO-1 mRNA expression levels on day 7 after SAH (p = 0.059, n = 50), an effect that could not be observed anymore by day 14 (p = 0.875, n = 19). b Irrespective of sonographic vasospasm, patients with confirmed moderate or severe vasospasm on CT angiography also showed significantly lower HO-1 mRNA expression levels on day 7 after SAH (p = 0.010, n = 53), but not on day 14 (p = 0.287, n = 21). c Patients with DCI had significantly higher HO-1 mRNA expression levels on day 14 after SAH than those without a DCI diagnosis (p = 0.009, n = 19). d CSF HO-1 expression levels on day 14 were significantly higher in patients that developed vasospasm and DCI than in those that developed vasospasm but no DCI (n = 17, p = 0.002). Data are plotted as median with interquartile range and minimum to maximum. *p < 0.05, **p < 0.01. CSF cerebrospinal fluid, CT computed tomography, DCI delayed cerebral ischemia, HO-1 hemoxygenase-1, mRNA messenger ribonucleic acid, VSP vasospasm, SAH subarachnoid hemorrhage
Predictive performance of CSF HO-1 expression regarding vasospasm and DCI. a For the presence of vasospasm on CT angiography and HO-1 mRNA expression on day 7, ROC curve analysis showed an AUC of 0.73 (0.57–0.89) (p = 0.01). b AUC was 0.87 (0.69–1.0) for HO-1 expression level and presence of DCI on day 7 after SAH (p = 0.01). c In the subgroup of patients with sonographic vasospasm, ROC analysis showed an AUC of 0.94 (0.81–1.0) for presence of DCI when considering day 14 HO-1 expression levels (p = 0.0036). AUC area under the curve, CSF cerebrospinal fluid, CT computed tomography, DCI delayed cerebral ischemia, HO-1 hemoxygenase-1, mRS modified Rankin Scale, ROC receiver operating characteristic, SAH subarachnoid hemorrhage, VSP vasospasm
In contrast, patients with DCI had significantly higher HO-1 mRNA expression levels on day 14 post SAH, compared with patients without a DCI diagnosis [HO-1 expression level 1777.0 (1348.0–1988.0) vs. 410.0 (90.48–755.4) (fold induction vs. control), n = 19, p = 0.009, Fig. 6c]. ROC curve AUC was 0.87 with a 95% confidence interval of 0.69 to 1.0 (p = 0.01, Fig. 7b). At a cutoff value of > 1254, day 14 HO-1 expression levels featured a sensitivity of 83.33%, a specificity of 92.31%, a PPV of 48.51% and an NPV of 52.05%. Further analyzing patient subgroups, patients who developed sonographic vasospasm and DCI had significantly higher HO-1 expression levels on day 14 than those who developed vasospasm, but no DCI [HO-1 expression level 1777.0 (1348.0–1988.0) vs. 410.0 (106.1–679.9) (fold induction vs. control), n = 17 p = 0.002, Fig. 6d]. This subgroup of patients with sonographic vasospasm showed an AUC of 0.94 with a 95% CI of 0.81 to 1.0 (p = 0.004, Fig. 7c). At a cutoff > 857.6 (fold induction vs. control), sensitivity of HO-1 expression was 83.33%, specificity 100%, PPV 46.42%, and NPV 54.13%.
HO-1 mRNA expression levels did not differ between patients that died during hospital treatment and those that could be discharged from hospital (p = 0.592, n = 53 on day 7 after SAH, data not shown).
Discussion
The aim of this study was to examine whether HO-1 expression levels in CSF and blood could represent prognostic markers that would indicate disease course, association with SAH complications and short-term and long-term functional outcome as early as on day 1 after SAH. In this single-center biomarker observational study, we confirmed that HO-1 mRNA expression levels in the CSF of patients with SAH correlate with intracranial hematoma size. We demonstrated that HO-1 expression rises over the course of the study observation period (2 weeks) after the SAH event. This study’s results do not support prognostication of functional neurological outcome based on HO-1 expression levels. However, we found evidence that high CSF HO-1 expression levels after one week of treatment were associated with lesser incidence of vasospasm, while late HO-1 induction (on day 14 after SAH) was associated with higher incidence of DCI. Associations of HO-1 expression with vasospasm and DCI need to be viewed with caution however as the small group sizes in this study did not allow for the consideration of covariates such as WFNS, age, and hematoma volume.
Our findings indicate that the semiquantitative measurement of total intracranial hematoma volume on the initial CT scans is an accurate indicator of disease severity and also reflects short-term functional development. The measurement of intracranial hematoma volume on a continuous scale rather than the traditionally employed categorical scales such as the modified Fisher scale allowed for finer discrimination when examined in relation to HO-1 induction.
In this study, CSF HO-1 expression on days 7 and 14 after SAH significantly correlated with total hematoma volume, corroborating previous results obtained from a smaller subgroup of the same patient collective [19]. Preclinical studies have demonstrated upregulation of HO-1 expression as early as 7 h after ICH [35], whereas HO-1 expression peaked at around 3 to 7 days [36, 37]. The lack of association between CSF HO-1 expression on day 1 after SAH and hematoma volume in our patient collective may reflect the delayed peak in HO-1 expression/HO-1 activity after hemorrhage, in line with the existing preclinical data on ICH [24, 37, 38].
HO-1 expression levels in blood leukocytes showed no association with CSF HO-1 expression or functional outcome parameters. In general, blood analyses have the distinct advantage of being far more readily available than CSF samples throughout the disease course and in a greater number of patients with SAH. The lack of correlation between CSF and blood leukocyte HO-1 expression levels regarding both the extent and temporal dynamic of HO-1 induction points to central nervous system microglia as being the principal mediators of HO-1 induction. This supports the hypothesis that upregulation of HO-1 expression following SAH is confined and thus specific to the central nervous system and does not involve systemic immune responses.
Preclinical models of ICH have demonstrated that the temporal course of HO-1 induction may be crucial regarding its function; one study showed toxic effects of early HO-1 upregulation after ICH [38], whereas late sustained upregulation exerted neuroprotective effects, while others proposed antioxidant, protective properties of early HO-1 induction during the first days after experimental ICH and prooxidant, detrimental effects of late sustained HO-1 upregulation [36, 38]. Our clinical data suggest that there may be detrimental effects of late sustained HO-1 upregulation after SAH.
In the nonfavorable outcome group, day 14 CSF HO-1 levels were higher than in the favorable outcome group. In terms of predictive ability, the multiple logistic regression model that included CSF HO-1 day 14 expression levels as predictor variable was superior to the model without CSF HO-1 levels and to the model with CSF HO-1 day 7 levels. However, CSF HO-1 day 14 expression levels did not reach significance as predictor variable in the multivariate model and WFNS could not be included in the model because of multicollinearity, limiting clinical applicability. It must be considered that this study was not powered to identify a nonassociation of HO-1 expression levels and clinical outcome, so that these results need to be viewed with caution. It must be taken into account that the number of patients for whom CSF samples were available on day 14 was limited (n = 21) and that those patients were more severely affected than the overall study population (Hunt & Hess Grade 3.45 vs. 3.2 in the overall study population, mRS at discharge 4.25 vs. 3.5, 1-year mRS 3.2 vs. 2.8, total hematoma volume 33.02 vs. 22.8 cm3). It is likely that higher HO-1 mRNA expression levels in patients with unfavorable outcome are an expression of disease severity and higher intracranial hematoma burden that mediates prolonged HO-1 induction. Rising HO-1 induction over the course of multiple weeks could be an indicator of ongoing microglial activation and thus neuroinflammation as one of the mediators of poor outcome in this patient subgroup. In a homogenous patient population consisting of patients with exclusively Fisher Grade III SAH, high HO-1 protein levels in the CSF on day 7 after SAH were associated with more unfavorable 3-month outcome [39]. Our data showed an association of higher HO-1 expression levels on day 14, but not day 7, with more unfavorable functional outcome. Considering the homogeneity of this study population further suggests that HO-1 induction has a component that may be independent of disease severity, although these results warrant confirmation in a larger study population.
EVD-associated complications occurred in 13.6% of the study population (ventriculitis: n = 5, intraparenchymal hematoma: n = 4). It must be taken into account that management-associated complications may influence functional short-term and long-term outcome and thus limit interpretability of outcome data.
The relationship between sonographic or angiographic vasospasm and delayed cerebral ischemia remains ambiguous, with DCI (even if showing a distinct vascular distribution pattern) not necessarily being associated with manifest vasospasm and vice versa [5]. Therefore, associations between the occurrence of DCI and vasospasm have to be made with caution.
In our study population, patients with sonographic or CT angiographic vasospasm showed lower CSF HO-1 levels on day 7, but not on day 14 after SAH, suggesting that early HO-1 induction may exert a protective effect on the development of vasospasm. In contrast, patients who developed DCI during the disease course had significantly higher HO-1 expression levels on day 14 after SAH, independent of the presence of sonographic vasospasm. However, HO-1 expression levels did not have sufficient predictive power with regards to vasospasm and DCI, limiting clinical applicability. As high HO-1 expression on day 14 was also associated with unfavorable functional outcome as discussed above, it should be further investigated whether prolonged HO-1 induction in the CSF could be an indicator of sustained neuroinflammation and thus increased neuronal injury, despite necessity of sufficient HO-1 induction for hematoma clearance.
In patients with SAH and vasospasm, HO-1 protein has been found to be increased compared to the CSF of patients with SAH without vasospasm [40]. The authors propose that increased HO-1 activity leads to increased formation of bilirubin and bilirubin oxidation products (BOXes) which increase the likelihood of vasospasm because of oxidative stress, despite availability of the same amount of substrate (hemoglobin) in the CSF. However, while CSF bilirubin concentration was demonstrated over a period of 10 days after SAH, this study did not specify the time-point of HO-1 determination in the CSF, so that taking a possible temporal induction pattern into account, it is unclear whether our data are contradictive.
Conclusions
Our study confirms that there is a distinct temporal induction pattern of HO-1 expression in the CSF of patients with SAH with an extent dependent on intracranial hematoma burden that cannot be observed in peripheral blood. HO-1 expression levels over the first week after SAH were unable to predict short- and long-term functional outcome. Patients with vasospasm showed lower CSF HO-1 expression levels on day 7, whereas late sustained HO-1 expression was associated with higher incidence of DCI, pointing to possible detrimental effects of sustained HO-1 expression during the second week after the SAH event. However, the small sample size of CSF samples on day 14 after SAH necessitates further validation in a larger study population.
References
Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635–42.
de Oliveira Manoel AL, Mansur A, Silva GS, Germans MR, Jaja BNR, Kouzmina E, et al. Functional outcome after poor-grade subarachnoid hemorrhage: a single-center study and systematic literature review. Neurocrit Care. 2016;25:338–50.
Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke U S. 2010;41:e519-536.
Ayling OGS, Ibrahim GM, Alotaibi NM, Gooderham PA, Macdonald RL. Dissociation of early and delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. Stroke. 2016;47:2945–51.
Vergouwen MDI, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42:924–9.
Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011;10:618–25.
Østergaard L, Aamand R, Karabegovic S, Tietze A, Blicher JU, Mikkelsen IK, et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013;33:1825–37.
Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:17–35.
Budohoski KP, Czosnyka M, Kirkpatrick PJ, Smielewski P, Steiner LA, Pickard JD. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat Rev Neurol. 2013;9:152–63.
Akamatsu Y, Pagan VA, Hanafy KA. The role of TLR4 and HO-1 in neuroinflammation after subarachnoid hemorrhage. J Neurosci Res. 2020;98:549–56.
Gomes JA, Selim M, Cotleur A, Hussain MS, Toth G, Koffman L, et al. Brain iron metabolism and brain injury following subarachnoid hemorrhage: iCeFISH-Pilot (CSF Iron in SAH). Neurocrit Care. 2014;21:285–93.
Reynolds RA, Amin SN, Jonathan SV, Tang AR, Lan M, Wang C, et al. Hyperoxemia and cerebral vasospasm in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2021;35(1):30–8.
Li X, Liu W, Li R, Guo S, Fan H, Wei B, et al. TSG-6 attenuates oxidative stress-induced early brain injury in subarachnoid hemorrhage partly by the HO-1 and Nox2 pathways. J Stroke Cerebrovasc Dis. 2020;29:104986.
Robinson SR, Dang TN, Dringen R, Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Rep. 2009;14:228–35.
Kwon KJ, Kim JN, Kim MK, Kim SY, Cho KS, Jeon SJ, et al. Neuroprotective effects of valproic acid against hemin toxicity: possible involvement of the down-regulation of heme oxygenase-1 by regulating ubiquitin–proteasomal pathway. Neurochem Int. 2013;62:240–50.
Lin S, Yin Q, Zhong Q, Lv F-L, Zhou Y, Li J-Q, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflamm. 2012;9:548.
Turner CP, Panter SS, Sharp FR. Anti-oxidants prevent focal rat brain injury as assessed by induction of heat shock proteins (HSP70, HO-1/HSP32, HSP47) following subarachnoid injections of lysed blood. Mol Brain Res. 1999;65:87–102.
Schallner N, Lieberum J-L, Gallo D, LeBlanc RH, Fuller PM, Hanafy KA, et al. Carbon monoxide preserves circadian rhythm to reduce the severity of subarachnoid hemorrhage in mice. Stroke. 2017;48:2565–73.
Kaiser S, Frase S, Selzner L, Lieberum J-L, Wollborn J, Niesen W-D, et al. Neuroprotection after hemorrhagic stroke depends on cerebral heme oxygenase-1. Antioxidants. 2019;8:496.
Schallner N, Pandit R, LeBlanc R, Thomas AJ, Ogilvy CS, Zuckerbraun BS, et al. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J Clin Invest. 2015;125:2609–25.
Ogawa T, Hänggi D, Wu Y, Michiue H, Tomizawa K, Ono S, et al. Protein therapy using heme-oxygenase-1 fused to a polyarginine transduction domain attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31:2231–42.
Zhang F, Wang S, Zhang M, Weng Z, Li P, Gan Y, et al. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke. 2012;43:1390–7.
Matz P, Turner C, Weinstein PR, Massa SM, Panter SS, Sharp FR. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res. 1996;713:211–22.
Liu Y, Zhang Z, Luo B, Schluesener HJ, Zhang Z. Lesional accumulation of heme oxygenase-1+ microglia/macrophages in rat traumatic brain injury. NeuroReport. 2013;24:281–6.
Chaudhry SR, Güresir A, Stoffel-Wagner B, Fimmers R, Kinfe TM, Dietrich D, et al. Systemic high-mobility group box-1: a novel predictive biomarker for cerebral vasospasm in aneurysmal subarachnoid hemorrhage*. Crit Care Med. 2018;46:e1023–8.
Wang L, Gao Z. Expression of MMP-9 and IL-6 in patients with subarachnoid hemorrhage and the clinical significance. Exp Ther Med. 2018;15:1510–4.
Wu W, Guan Y, Zhao G, Fu X-J, Guo T-Z, Liu Y-T, et al. Elevated IL-6 and TNF-α levels in cerebrospinal fluid of subarachnoid hemorrhage patients. Mol Neurobiol. 2016;53:3277–85.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, et al. Prediction of symptomatic vasospasmafter subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59:21–7.
Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21:1156–61.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8.
Kumar G, Shahripour RB, Harrigan MR. Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. JNS. 2016;124:1257–64.
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5.
Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–6.
Chen-Roetling J, Song W, Schipper HM, Regan CS, Regan RF. Astrocyte overexpression of heme oxygenase-1 improves outcome after intracerebral hemorrhage. Stroke. 2015;46:1093–8.
Wang G, Yang Q, Li G, Wang L, Hu W, Tang Q, et al. Time course of heme oxygenase-1 and oxidative stress after experimental intracerebral hemorrhage. Acta Neurochir. 2011;153:319–25.
Chen M, Regan RF. Time course of increased heme oxygenase activity and expression after experimental intracerebral hemorrhage: correlation with oxidative injury. J Neurochem. 2007;103:2015–21.
Zhang Z, Song Y, Zhang Z, Li D, Zhu H, Liang R, et al. Distinct role of heme oxygenase-1 in early- and late-stage intracerebral hemorrhage in 12-month-old mice. J Cereb Blood Flow Metab. 2017;37:25–38.
Wang K-C, Tang S-C, Lee J-E, Lai D-M, Huang S-J, Hsieh S-T, et al. Prognostic value of intrathecal heme oxygenase–1 concentration in patients with Fisher Grade III aneurysmal subarachnoid hemorrhage. J Neurosurg. 2014;121:1388–93.
Pyne-Geithman GJ, Morgan CJ, Wagner K, Dulaney EM, Carrozzella J, Kanter DS, et al. Bilirubin production and oxidation in CSF of patients with cerebral vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:1070–7.
Funding
Open Access funding enabled and organized by Projekt DEAL. German Research Foundation Grant to NS: SCHA 1838/4–1. Funded by the Berta-Ottenstein-Programme for Advanced Clinician Scientists, Faculty of Medicine, University of Freiburg (NS).
Author information
Authors and Affiliations
Contributions
Study concept and design: NS. Acquisition of data: MS, SK, SF, LS, and NAF. Statistical analysis and interpretation of data: SF and NS. Funding acquisition: NS. Project administration: NS, SK, and WDN. Drafting of the articlet: SF and NS. Critical revision of the article for intellectual content: all authors. Study supervision: NS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval/informed consent
The study protocol was approved by the Institutional Ethics Committee of the University of Freiburg (Protocol No. 293/15). It has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent has been obtained from all individual participants included in this study or from their legal representatives according to federal regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frase, S., Steimer, M., Selzner, L. et al. Temporal Expression Pattern of Hemoxygenase-1 Expression and Its Association with Vasospasm and Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 36, 279–291 (2022). https://doi.org/10.1007/s12028-021-01299-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01299-w