Abstract
Background
Mitochondrial dysfunction is related to brain ischemic injury and neural cell death. However, little is known about the association between mitochondrial dysfunction of cerebrospinal fluid (CSF) and delayed cerebral ischemia (DCI) following subarachnoid hemorrhage (SAH). The objective of this study was to investigate whether extracellular CSF mitochondria might serve as a potential biomarker for DCI.
Methods
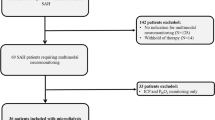
CSF samples were serially collected at 1, 3, and 5 days following SAH in 33 patients (DCI, n = 12; and non-DCI, n = 21) who underwent coil embolization. To monitor mitochondrial membrane potentials, JC-1 dye was used. The ratio (red/green) of JC-1 was considered as an indicator of intact mitochondrial membrane potential. Flow cytometry was done to analyze extracellular mitochondria particles and their possible cellular origins.
Results
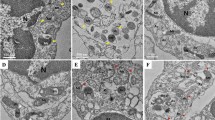
DCI patients had lower JC-1 red/green ratios than non-DCI patients at 1 day (3.35 [3.20–3.75] vs. 3.70 [3.40–3.95] in non-DCI) and 3 days (4.65 [4.45–5.00] vs. 5.10 [4.65–5.30] in non-DCI) after SAH. At 5 days after SAH, JC-1 red/green ratio was significantly lower in DCI than that in non-DCI (3.05 [2.90–3.35] vs. 4.20 [4.10–4.50]; p < 0.01) patients. DCI patients had a higher percentage of vWF-positive mitochondria (40.10% [38.25%–44.90%] vs. 30.20% [25.70%–36.68%]) and a lower percentage of GLAST-positive mitochondria particles (26.85% [17.10%–30.00%] vs. 31.60% [26.70%–35.00%]) than non-DCI patients. However, there was no significant difference in CD45-positive (p = 0.369) or CD41/61-positive mitochondrial particles (p = 0.155) between the two groups of patients.
Conclusions
Mitochondrial membrane potential could be a marker of DCI. JC-1 ratios seemed to be able to predict future DCI onset. Further studies are needed to determine detailed mechanisms of extracellular mitochondria-mediated cell-to-cell signals in DCI.


Similar content being viewed by others
References
Chou SH, Lan J, Esposito E, et al. Extracellular mitochondria in cerebrospinal fluid and neurological recovery after subarachnoid hemorrhage. Stroke. 2017;48:2231–7.
Miliotis S, Nicolalde B, Ortega M, Yepez J, Caicedo A. Forms of extracellular mitochondria and their impact in health. Mitochondrion. 2019;48:16–30.
Boudreau LH, Duchez AC, Cloutier N, et al. Platelets release mitochondria serving as substrate for bactericidal group iia-secreted phospholipase a2 to promote inflammation. Blood. 2014;124:2173–83.
Hayakawa K, Bruzzese M, Chou SH, Ning M, Ji X, Lo EH. Extracellular mitochondria for therapy and diagnosis in acute central nervous system injury. JAMA Neurol. 2018;75:119–22.
Al-Shahi R, White PM, Davenport RJ, Lindsay KW. Subarachnoid haemorrhage. BMJ. 2006;333:235–40.
Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413–8.
Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97:14–37.
Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5.
Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth. 2012;109:315–29.
Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 2016;20:277.
Hong EP, Kim BJ, Cho SS, et al. Genomic variations in susceptibility to intracranial aneurysm in the Korean population. J Clin Med. 2019;8:275.
Kim BJ, Kim Y, Hong EP, et al. Correlation between altered DNA methylation of intergenic regions of ITPR3 and development of delayed cerebral ischemia in subarachnoid hemorrhage patients. World Neurosurg. 2019;13:449–56.
Cho YD, Kim SE, Lim JW, Choi HJ, Cho YJ, Jeon JP. Protected versus unprotected carotid artery stenting: meta-analysis of the current literature. J Korean Neurosurg Soc. 2018;61:458–66.
Kim CH, Jeon JP, Kim SE, Choi HJ, Cho YJ. Endovascular treatment with intravenous thrombolysis versus endovascular treatment alone for acute anterior circulation stroke: a meta-analysis of observational studies. J Korean Neurosurg Soc. 2018;61:467–73.
Kim BJ, Kim Y, Kim SE, Jeon JP. Study of correlation between hp alpha1 expression of haptoglobin 2-1 and clinical course in aneurysmal subarachnoid hemorrhage. World Neurosurg. 2018;117:e221–7.
Jeon JS, Sheen SH, Hwang G, Kang SH, Heo DH, Cho YJ. Intravenous magnesium infusion for the prevention of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc. 2012;52:75–9.
Cho SS, Kim SE, Kim HC, Kim WJ, Jeon JP. Clazosentan for aneurysmal subarachnoid hemorrhage: an updated meta-analysis with trial sequential analysis. World Neurosurg. 2019;123:418–24.
Kim W, Lee SH, Ahn YJ, et al. A label-free cellulose SERS biosensor chip with improvement of nanoparticle-enhanced LSPR effects for early diagnosis of subarachnoid hemorrhage-induced complications. Biosens Bioelectron. 2018;111:59–65.
Wong FW. Cerebrospinal fluid collection: a comparison of different collection sites on the external ventricular drain. Dynamics. 2011;22:19–24.
Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 2018;9:924–37.
Jeon JS, Ahn JH, Moon YJ, et al. Expression of cellular retinoic acid-binding protein-i (crabp-i) in the cerebrospinal fluid of adult onset moyamoya disease and its association with clinical presentation and postoperative haemodynamic change. J Neurol Neurosurg Psychiatry. 2014;85:726–31.
Peeyush Kumar T, McBride DW, Dash PK, Matsumura K, Rubi A, Blackburn SL. Endothelial cell dysfunction and injury in subarachnoid hemorrhage. Mol Neurobiol. 2019;56:1992–2006.
Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–25.
Clower BR, Yamamoto Y, Cain L, Haines DE, Smith RR. Endothelial injury following experimental subarachnoid hemorrhage in rats: effects on brain blood flow. Anat Rec. 1994;240:104–14.
Comair YG, Schipper HM, Brem S. The prevention of oxyhemoglobin-induced endothelial and smooth muscle cytoskeletal injury by deferoxamine. Neurosurgery. 1993;32:58–64.
Kasuya H, Weir BK, White DM, Stefansson K. Mechanism of oxyhemoglobin-induced release of endothelin-1 from cultured vascular endothelial cells and smooth-muscle cells. J Neurosurg. 1993;79:892–8.
Thampatty BP, Sherwood PR, Gallek MJ, et al. Role of endothelin-1 in human aneurysmal subarachnoid hemorrhage: associations with vasospasm and delayed cerebral ischemia. Neurocrit Care. 2011;15:19–27.
Bendok BR, Getch CC, Malisch TW, Batjer HH. Treatment of aneurysmal subarachnoid hemorrhage. Semin Neurol. 1998;18:521–31.
Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–46.
Barsoum MJ, Yuan H, Gerencser AA, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–11.
Motori E, Puyal J, Toni N, et al. Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab. 2013;18:844–59.
Blackburn D, Sargsyan S, Monk PN, Shaw PJ. Astrocyte function and role in motor neuron disease: a future therapeutic target? Glia. 2009;57:1251–64.
Owens K, Park JH, Gourley S, Jones H, Kristian T. Mitochondrial dynamics: cell-type and hippocampal region specific changes following global cerebral ischemia. J Bioenerg Biomembr. 2015;47:13–31.
Liu K, Ji K, Guo L, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–8.
Hayakawa K, Esposito E, Wang X, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–5.
Kramer AH, Hehir M, Nathan B, et al. A comparison of 3 radiographic scales for the prediction of delayed ischemia and prognosis following subarachnoid hemorrhage. J Neurosurg. 2008;109:199–207.
Komotar RJ, Hahn DK, Kim GH, et al. The impact of microsurgical fenestration of the lamina terminalis on shunt-dependent hydrocephalus and vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2008;62:123–32.
Acknowledgements
None.
Funding
This study received funding from the National IT Industry Promotion Agency (R-20190315-004075) and Hallym University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no financial conflicts of interest.
Ethical approval
This study was approved by the hospital institutional review board (No. 2016-3, 2017-9 and 2018-6).
Informed consent
Informed consent was received from patients or their relatives.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Youn, D.H., Kim, B.J., Kim, Y. et al. Extracellular Mitochondrial Dysfunction in Cerebrospinal Fluid of Patients with Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 33, 422–428 (2020). https://doi.org/10.1007/s12028-019-00895-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00895-1