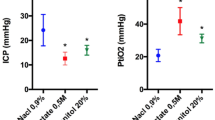
Abstract
Background
Spontaneous intracerebral hemorrhage (ICH) leaves most survivors dependent at follow-up. The importance of promoting M2-like microglial responses is increasingly recognized as a key element to ameliorate brain injury following ICH. The osmotherapeutic agents, mannitol and hypertonic saline (HTS), which are routinely used to reduce intracranial pressure, have been shown to reduce neuroinflammation in experimental ischemic and traumatic brain injury, but anti-inflammatory effects of osmotherapies have not been investigated in ICH.
Methods
We studied the effects of iso-osmotic mannitol and HTS in rat models of ICH utilizing high-dose and moderate-dose collagenase injections into the basal ganglia, associated with high and low mortality, respectively. We studied the effects of osmotherapies, first given 5 h after ICH induction, and then administered every 12 h thereafter (4 doses total). Immunohistochemistry was used to quantify microglial activation and polarization.
Results
Compared to controls, mannitol and HTS increased plasma osmolarity 1 h after infusion (301 ± 1.5, 315 ± 4.2 and 310 ± 2.0 mOsm/kg, respectively), reduced mortality at 48 h (82, 36 and 53%, respectively), and reduced hemispheric swelling at 48 h (32, 21, and 17%, respectively). In both perihematomal and contralateral tissues, mannitol and HTS reduced activation of microglia/macrophages (abundance and morphology of Iba1 + cells), and in perihematomal tissues, they reduced markers of the microglia/macrophage M1-like phenotype (nuclear p65, TNF, and NOS2), increased markers of the microglia/macrophage M2-like phenotype (arginase, YM1, and pSTAT3), and reduced infiltration of CD45 + cells.
Conclusions
Repeated dosing of osmotherapeutics at regular intervals may be a useful adjunct to reduce neuroinflammation following ICH.






Similar content being viewed by others
References
Urday S, Kimberly WT, Beslow LA, et al. Targeting secondary injury in intracerebral haemorrhage–perihaematomal oedema. Nat Rev Neurol. 2015;11:111–22.
Torre-Healy A, Marko NF, Weil RJ. Hyperosmolar therapy for intracranial hypertension. Neurocrit Care. 2012;17:117–30.
Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–77.
Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388.
Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13:420–33.
Durafourt BA, Moore CS, Zammit DA, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–27.
Junger WG, Hoyt DB, Davis RE, et al. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Investig. 1998;101:2768–79.
Junger WG, Rhind SG, Rizoli SB, et al. Resuscitation of traumatic hemorrhagic shock patients with hypertonic saline-without dextran-inhibits neutrophil and endothelial cell activation. Shock. 2012;38:341–50.
Shields CJ, O’Sullivan AW, Wang JH, Winter DC, Kirwan WO, Redmond HP. Hypertonic saline enhances host response to bacterial challenge by augmenting receptor-independent neutrophil intracellular superoxide formation. Ann Surg. 2003;238:249–57.
Rizoli SB, Rhind SG, Shek PN, et al. The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: a randomized, controlled, double-blinded trial. Ann Surg. 2006;243:47–57.
Huang LQ, Zhu GF, Deng YY, et al. Hypertonic saline alleviates cerebral edema by inhibiting microglia-derived TNF-alpha and IL-1beta-induced Na–K–Cl Cotransporter up-regulation. J Neuroinflamm. 2014;11:102.
Soustiel JF, Vlodavsky E, Zaaroor M. Relative effects of mannitol and hypertonic saline on calpain activity, apoptosis and polymorphonuclear infiltration in traumatic focal brain injury. Brain Res. 2006;1101:136–44.
Kumasaka K, Marks JA, Eisenstadt R, et al. In vivo leukocyte-mediated brain microcirculatory inflammation: a comparison of osmotherapies and progesterone in severe traumatic brain injury. Am J Surg. 2014;208:961–8.
Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283:230–2.
MacLellan CL, Silasi G, Poon CC, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–25.
Qureshi AI, Wilson DA, Traystman RJ. Treatment of elevated intracranial pressure in experimental intracerebral hemorrhage: comparison between mannitol and hypertonic saline. Neurosurgery. 1999;44:1055–63.
Mirski AM, Denchev ID, Schnitzer SM, Hanley FD. Comparison between hypertonic saline and mannitol in the reduction of elevated intracranial pressure in a rodent model of acute cerebral injury. J Neurosurg Anesthesiol. 2000;12:334–44.
Bermueller C, Thal SC, Plesnila N, Schmid-Elsaesser R, Kreimeier U, Zausinger S. Hypertonic fluid resuscitation from subarachnoid hemorrhage in rats: a comparison between small volume resuscitation and mannitol. J Neurol Sci. 2006;241:73–82.
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–84.
Marks JA, Li S, Gong W, et al. Similar effects of hypertonic saline and mannitol on the inflammation of the blood-brain barrier microcirculation after brain injury in a mouse model. J Trauma Acute Care Surg. 2012;73:351–7.
MacLellan CL, Auriat AM, McGie SC, et al. Gauging recovery after hemorrhagic stroke in rats: implications for cytoprotection studies. J Cereb Blood Flow Metab. 2006;26:1031–42.
Patel AD, Gerzanich V, Geng Z, Simard JM. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol. 2010;69:1177–90.
Verdaasdonk JS, Lawrimore J, Bloom K. Determining absolute protein numbers by quantitative fluorescence microscopy. Methods Cell Biol. 2014;123:347–65.
Wasserman JK, Schlichter LC. Minocycline protects the blood-brain barrier and reduces edema following intracerebral hemorrhage in the rat. Exp Neurol. 2007;207:227–37.
Kurland DB, Gerzanich V, Karimy JK, et al. The Sur1-Trpm4 channel regulates NOS2 transcription in TLR4-activated microglia. J Neuroinflamm. 2016;13:130.
Altin JG, Sloan EK. The role of CD45 and CD45-associated molecules in T cell activation. Immunol Cell Biol. 1997;75:430–45.
Ouriel K, Ginsburg ME, Patti CS, Pearce FJ, Hicks GL. Preservation of myocardial function with mannitol reperfusate. Circulation. 1985;72:II254–8.
Yilmaz N, Dulger H, Kiymaz N, Yilmaz C, Gudu BO, Demir I. Activity of mannitol and hypertonic saline therapy on the oxidant and antioxidant system during the acute term after traumatic brain injury in the rats. Brain Res. 2007;1164:132–5.
Kim H, Seo JY, Kim KH. Effects of mannitol and dimethylthiourea on helicobacter pylori-induced IL-8 production in gastric epithelial cells. Pharmacology. 1999;59:201–11.
Kim JH, Kim YS, Kim SH, et al. Contralateral hemispheric brain atrophy after primary intracerebral hemorrhage. World Neurosurg. 2017;102:56–64.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–60.
Gigliuto CM, Stone KE, Algus M. The use of mannitol in intracerebral bleeds in the medical ICU. N J Med J Med Soc N J. 1991;88:48–51.
Bereczki D, Mihalka L, Szatmari S, et al. Mannitol use in acute stroke: case fatality at 30 days and 1 year. Stroke. 2003;34:1730–5.
Kalita J, Misra UK, Ranjan P, Pradhan PK, Das BK. Effect of mannitol on regional cerebral blood flow in patients with intracerebral hemorrhage. J Neurol Sci. 2004;224:19–22.
Misra UK, Kalita J, Ranjan P, Mandal SK. Mannitol in intracerebral hemorrhage: a randomized controlled study. J Neurol Sci. 2005;234:41–5.
Bereczki D, Fekete I, Prado GF, Liu M. Mannitol for acute stroke. Cochrane Database Syst Rev. 2007; CD001153.
Wu J, Yang S, Hua Y, Liu W, Keep RF, Xi G. Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Acta neurochirurgica Suppl. 2010;106:147–50.
Wasserman JK, Schlichter LC. Neuron death and inflammation in a rat model of intracerebral hemorrhage: effects of delayed minocycline treatment. Brain Res. 2007;1136:208–18.
Kobayashi K, Imagama S, Ohgomori T, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4:e525.
Jeong GR, Im SK, Bae YH, et al. Inflammatory signals induce the expression of tonicity-responsive enhancer binding protein (TonEBP) in microglia. J Neuroimmunol. 2016;295–296:21–9.
Funding
This work was supported in part by a grant to DLS from the Department of Anesthesia. JMS is supported by a grant from the National Heart, Lung, and Blood Institute (HL082517).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schreibman, D.L., Hong, C.M., Keledjian, K. et al. Mannitol and Hypertonic Saline Reduce Swelling and Modulate Inflammatory Markers in a Rat Model of Intracerebral Hemorrhage. Neurocrit Care 29, 253–263 (2018). https://doi.org/10.1007/s12028-018-0535-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-018-0535-7