Abstract
Background
The optimal timing of tracheostomy placement in acutely brain-injured patients, who generally require endotracheal intubation for airway protection rather than respiratory failure, remains uncertain. We systematically reviewed trials comparing early tracheostomy to late tracheostomy or prolonged intubation in these patients.
Methods
We searched 5 databases (from inception to April 2015) to identify randomized controlled trials comparing early tracheostomy (≤10 days of intubation) with late tracheostomy (>10 days) or prolonged intubation in acutely brain-injured patients. We contacted the principal authors of included trials to obtain subgroup data. Two reviewers extracted data and assessed risk of bias. Outcomes included long-term mortality (primary), short-term mortality, duration of mechanical ventilation, complications, and liberation from ventilation without a tracheostomy. Meta-analyses used random-effects models.
Results
Ten trials (503 patients) met selection criteria; overall study quality was moderate to good. Early tracheostomy reduced long-term mortality (risk ratio [RR] 0.57. 95 % confidence interval (CI), 0.36–0.90; p = 0.02; n = 135), although in a sensitivity analysis excluding one trial, with an unclear risk of bias, the significant finding was attenuated (RR 0.61, 95 % CI, 0.32–1.16; p = 0.13; n = 95). Early tracheostomy reduced duration of mechanical ventilation (mean difference [MD] −2.72 days, 95 % CI, −1.29 to −4.15; p = 0.0002; n = 412) and ICU length of stay (MD −2.55 days, 95 % CI, −0.50 to −4.59; p = 0.01; n = 326). However, early tracheostomy did not reduce short-term mortality (RR 1.25; 95 % CI, 0.68–2.30; p = 0.47 n = 301) and increased the probability of ever receiving a tracheostomy (RR 1.58, 95 % CI, 1.24–2.02; 0 < 0.001; n = 377).
Conclusions
Performing an early tracheostomy in acutely brain-injured patients may reduce long-term mortality, duration of mechanical ventilation, and ICU length of stay. However, waiting longer leads to fewer tracheostomy procedures and similar short-term mortality. Future research to explore the optimal timing of tracheostomy in this patient population should focus on patient-centered outcomes including patient comfort, functional outcomes, and long-term mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most patients suffering from significant acute brain injury require airway protection and mechanical ventilation in the acute period, generally with an endotracheal tube. This translaryngeal approach, which makes oral care, communication, and feeding challenging, is usually poorly tolerated unless sedation is administered. Thus, clinicians often consider exchanging this tube for a tracheostomy. The anticipated benefits of tracheostomy include enhanced comfort, improved pulmonary hygiene, and decreased sedation requirements, all of which should accelerate liberation from the ventilator and discharge from critical care. However, this procedure is not without risks. Early complications include esophageal and airway injury, stomal bleeding, and barotrauma; and delayed complications include infections, tracheomalacia, tracheal stenosis, and tracheoinnominate fistula.
Observational studies conducted in acutely brain-injured patients suggest that early tracheostomy may be associated with lower in-hospital morbidity and improved clinical outcomes [1–5], but results are inconsistent and subject to confounding by indication. Systematic reviews of randomized controlled trials (RCTs) in mixed critical care populations have generally not found benefit from early tracheostomy (generally defined as occurring within 10 days of intubation) [6–8]. These results may not apply to acutely brain-injured patients, who typically require airway protection for depressed airway reflexes rather than respiratory failure. Consequently, early tracheostomy in brain-injured patients may expedite liberation from the ventilator while maintaining airway patency [9].
Our primary objective was to systematically review all RCTs and quasi-randomized controlled trials comparing early tracheostomy to late tracheostomy or prolonged intubation in acutely brain-injured patients to determine effects on long-term, all-cause mortality.
Methods
We conducted this systematic review using a predefined protocol according to current standards [10] and adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [11, 12]. The Research Ethics Board of Sunnybrook Health Sciences Centre reviewed the study and deemed it exempt from review. Our protocol was registered with PROSPERO: International prospective register of systematic reviews (No: CRD42014010405); available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014010405.
Search Strategy
We searched MEDLINE (1966–April 2015), EMBASE (1974–April 2015), CINAHL (1982–April 2015), CENTRAL (April 2015), and Web of Science (April 2015) (details in Supplemental Digital Content—Search Strategy) to identify RCTs and quasi-randomized trials comparing early tracheostomy with late tracheostomy or prolonged intubation. MEDLINE and EMBASE citations were limited to RCTs using sensitive strategies [13, 14]. To search gray literature, we hand-searched conference proceedings of the American Thoracic Society (1994–2015), Society of Critical Care Medicine (1994–2015), European Society of Intensive Care Medicine (1994–2015), American College of Chest Physicians (1994–2015), and the International Symposium on Intensive Care and Emergency Medicine (1999–2015). We contacted primary investigators of eligible abstracts for further information. Finally, we searched for unpublished and ongoing trials in http://www.clinicaltrials.gov and http://www.controlled-trials.com. No language restrictions were applied.
Trial Selection
Two reviewers (V.A.M., A.S.A.) independently screened studies for inclusion, retrieved potentially relevant studies, and decided on study eligibility. We selected RCTs and quasi-randomized trials comparing early tracheostomy (performed within 10 days of intubation) to late tracheostomy (performed after the 10th day of intubation) or prolonged intubation in intubated adults with acute brain injury (traumatic brain injury, aneurysmal subarachnoid hemorrhage, acute ischemic stroke, spontaneous intracerebral hemorrhage, postcraniotomy, global cerebral anoxia due to cardiac arrest, status epilepticus, meningitis, encephalitis, or cerebral abscess). Quasi-randomized trials included those allocating participants to treatment arms by alternate or predictable assignment. We included studies that reported on all-cause mortality at any time.
Data Abstraction
Two reviewers (V.A.M., A.S.A.) independently abstracted data and methodological characteristics from the included trials using a standard form (Supplemental Digital Content). Disagreements were resolved by consensus and, if necessary, in consultation with a third reviewer (D.C.S.). We contacted investigators to obtain subgroup data for acutely brain-injured patients from trials conducted in the general critical care population (Supplemental Digital Content).
Risk of Bias
Two reviewers (V.A.M. and A.S.A.) independently assessed the risk of bias (selection, performance, detection, attrition, and reporting bias) for each included trial. Any disagreement was resolved through discussion with a third reviewer (D.C.S.). We used the Cochrane Collaboration’s tool [15] to assess risk of bias at the study level according to the following domains: random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting.
Outcomes
The primary outcome was long-term, all-cause mortality, defined as deaths reported at 6–12 months following acute brain injury. Secondary outcomes were short-term mortality, ICU mortality, hospital mortality, ICU length of stay, ventilator-associated pneumonia (VAP), duration of mechanical ventilation, duration of sedation, time to mobility, laryngotracheal complications (epiglottis, vocal cords, larynx, or subglottic ulceration and inflammation, or tracheostomy complications), and liberation from mechanical ventilation without a tracheostomy. Short-term mortality was defined as in-hospital mortality or, if not available, at 60 days after randomization. Three trials did not specify the timing of mortality assessment and were only included in the short-term mortality analysis [9, 16, 17].
Subgroup and Sensitivity Analyses
A priori subgroup analyses were conducted to explore heterogeneity in the primary and secondary outcomes of long- and short-term mortality: (a) year of publication (before vs. after median year); (b) size of trial (below vs. above median number of patients); (c) low risk versus non-low risk of bias; (d) timing of early tracheostomy (1–3 days vs. 4–10 days); (e) patient population (traumatic brain injury vs. mixed acute brain injuries); (f) control group (late tracheostomy vs. prolonged intubation only); and (g) type of tracheostomy (percutaneous vs. surgical). We also planned sensitivity analyses to explore the influence of analysis methods (per-protocol analysis vs. intention to treat), risk of bias, and exclusion of studies enrolling heterogeneous trauma patients on outcomes.
Quantitative Data Synthesis
We used random effects models [18] to calculate pooled estimates of effect sizes using Review Manager 5.3.5 software (Cochrane Collaboration, Oxford, UK). Pooled continuous-effect measures were expressed as mean differences (MD) and pooled dichotomous effect measures as risk ratios (RR), both with 95 % confidence intervals (CI). We performed a z test of interaction for all subgroup comparisons, which tests the null hypothesis that the treatment effects in each subgroup are the same. Our analyses adhered to the intention-to-treat principle. Trials with zero deaths in either treatment arm were included by adding 0.5 to each cell [19]. We assessed between-study statistical heterogeneity for each outcome using the I2 measure [20, 21], with suggested thresholds for low (25–49 %), moderate (50–74 %), and high (≥75 %) values of I2. We decided not to report meta-analyses in the presence of high statistical heterogeneity. To assess for publication bias, we examined a funnel plot of trial effect size versus trial precision [22].
Results
Literature Search
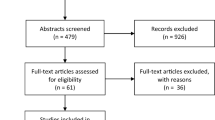
We identified 2275 citations from searches of electronic bibliographies and two citations from the gray literature. We retrieved 22 articles for detailed evaluation, 12 of which were excluded for the following reasons: inability to obtain acutely brain-injured subgroup data [23–26], incomplete data from published abstract [27], lack of acutely brain-injured patients (e.g. cardiac surgery, burns, and general medical patients) [28–31], and no mortality data [32–34] (Supplemental Digital Content—Tables 1 and 2). Ten trials enrolling 503 patients [9, 16, 17, 35–41] met the inclusion criteria for our review (Fig. 1; Table 1). The authors of 4 trials provided us with previously unpublished subgroup data for acutely brain-injured patients [35, 36, 40, 41]. We also contacted several trial investigators to clarify study procedures [9, 16, 17, 37, 39]. Four trials found in trial registries were actively recruiting patients or recently terminated (Supplemental Digital Content—Table 3).
Study Characteristics and Methodological Quality
Table 1 summarizes the study characteristics of 10 included randomized controlled trials (see Supplemental Digital Content—Table 4 for patient characteristics). The majority of trials performed early tracheostomies within the first 5 days [17, 35, 38–41], while only one trial used a timeframe up to 10 days [37]. The control group was late tracheostomy in five trials [16, 35–38], prolonged translaryngeal intubation in three trials [9, 40, 41], and late tracheostomy or prolonged intubation in two trials [17, 39]. Six trials exclusively enrolled patients with traumatic brain injury [9, 16, 17, 36, 38, 41], three trials included patients with mixed causes of acute brain injury [35, 37, 40], and one trial enrolled stroke patients only [39]. Studies used different definitions of VAP (Supplemental Digital Content—Table 5): Centers for Disease Control and Prevention (CDC) criteria [9, 16, 42], criteria closely aligned to CDC [17, 38], or American Thoracic Society consensus statement and guidelines [43, 44] for diagnosis of ICU-acquired pneumonia [40, 41]. Overall, the quality of the included trials was moderate to good (Supplemental Digital Content—Table 6 and Fig. 1). Visual inspection of the funnel plot for hospital mortality did not suggest publication bias.
Quantitative Data Synthesis
Primary Outcome
The primary outcome of long-term mortality was reported at 6 months [39, 41] and 1 year [35]; meta-analysis showed that early tracheostomy reduced long-term mortality (RR 0.57, 95 % CI, 0.36–0.90; p = 0.02; three trials; n = 135; 52 events; I2 2 %; Fig. 2, Table 2, Supplemental Digital Content—Table 7). In a sensitivity analysis excluding one trial [41] with an unclear risk of bias, the significant finding was attenuated (RR 0.61, 95 % CI, 0.32-1.16; p = 0.13; n = 95; two trials; I2 43 %). There were insufficient trials to perform other subgroup and sensitivity analyses.
Long-term mortality: Random-effects meta-analysis of early tracheostomy versus late tracheostomy or prolonged intubation on long-term mortality, expressed as the risk ratio (RR), with values more than 1 indicating increased mortality with early tracheostomy. Each black square and horizontal line denotes the point estimate and 95 % CI for each trial’s RR. The diamond signifies the pooled RR for all trials, with the center denoting the point estimate and the width the 95 % CI. Weight is the contribution of each study to the overall RR. Young et al. measured mortality at 1 year, Bösel et al. measured at 6 months, Fayed et al. recorded mortality until hospital discharge or death (whichever came first) for a maximum of 6 months. ET early tracheostomy group, LT/PI late tracheostomy or prolonged intubation, I 2 percentage of total variation across studies from between-study heterogeneity rather than chance, CI confidence interval
Secondary Outcomes
Short-Term Mortality
Seven trials (301 participants) provided data for all-cause, short-term mortality [9, 16, 17, 35, 37, 38, 40] that was reported at hospital discharge [35, 37, 38], 60 days [40], or presumed short-term mortality [9, 16, 17], in keeping with previous systematic reviews [8, 45]. Short-term mortality was similar between groups (RR 1.25, 95 % CI, 0.68–2.30; p = 0.47; seven trials; n = 301; 61 events; Table 2, Supplemental Digital Content - Fig. 2). Additional analyses (Table 3) showed similar effects in all subgroups examined; sensitivity analyses using per-protocol analysis for two trials [37, 38], intention-to-treat analysis assuming that all patients lost to follow-up died [37, 38], or removing one trial that enrolled general trauma patients [16] did not change the treatment effect (Table 3).
Hospital and ICU Mortality
Four trials (112 patients; 29 events) recorded mortality at hospital discharge [35, 37, 38] or 60 days [40] and found similar hospital mortality in both arms (RR 1.17, 95 % CI, 0.46, 2.94; p = 0·18; I2 38 %; Supplemental Digital Content—Fig. 3). Three trials (197 patients; 49 events) recorded mortality at ICU discharge [35, 39] or 28 days [36] and found lower ICU mortality with early tracheostomy (RR 0.46, 95 % CI, 0.24, 0.89; p = 0·02; I2 21 %; Supplemental Digital Content—Fig. 4).
Rate of Tracheostomies and Laryngotracheal Complications
Seven trials (377 patients; 277 tracheostomies) reported the number of patients undergoing tracheostomy procedures in both groups [16, 17, 35, 36, 38–40]. As expected, patients in the early tracheostomy group were more likely to undergo the procedure (RR, 1.58, 95 % CI, 1.24, 2.02; p < 0·001; n = 377; seven trials; I2 70 %; Supplemental Digital Content—Fig. 5). Pooled data from four studies (222 patients; 37 patients with complications) found no significant difference in laryngotracheal complications (RR 2.54, 95 % CI, 0.46–13.88; p = 0.28; I2 59 %; Supplemental Digital Content—Fig. 6) [9, 16, 39, 41].
Other Secondary Outcomes
Early tracheostomy reduced the mean duration of ventilation by 2.72 days (95 % CI, −1.29 to −4.15; p = 0.0002; I2 = 0 %; Supplemental Digital Content—Fig. 7) and the mean ICU length of stay by 2.55 days (95 % CI, −0.50 to −4.59; p = 0.01; I2 = 0 %; Supplemental Digital Content—Fig. 8). There was no effect on VAP (RR 0.89, 95 % CI, 0.65–1.21; p = 0.46; I2 54 %; Supplemental Digital Content—Fig. 9). Data on duration of sedation, length of hospital stay, and time to mobility were infrequently or nonuniformly reported, precluding meta-analysis. Furthermore, subgroup analyses regarding timing of early tracheostomy and type of tracheostomy could not be completed due to insufficient data.
Heterogeneity
Clinical heterogeneity among studies existed due to the inclusion of different brain injury etiologies, variable timing of early tracheostomy and mortality assessments, and variable risk of bias. However, all included studies were judged to be sufficiently similar to be pooled in meta-analyses. Statistical heterogeneity was low to moderate for all meta-analyses.
Discussion
Summary of Main Results
Our main finding of this systematic review is that early tracheostomy, compared with late tracheostomy or prolonged translaryngeal intubation, might lower ICU and long-term mortality. However, inferences are severely limited by the small number of studies and outcome events, and lack of consistency with the effect on mortality measured at hospital discharge or at other time points. Therefore, the potential to improve survival with early tracheostomy should be seen as hypothesis-generating. A strategy of early tracheostomy also reliably increases the proportion of patients undergoing the procedure and reduces duration of mechanical ventilation and ICU length of stay; the latter two outcomes may be of more interest to health systems and/or payers than to patients [46]. Considering the procedural risks, acutely brain-injured patients with uncertain or poor neurological prognosis may be better served by waiting longer before committing to the options of tracheostomy or primary extubation [47, 48].
Most of the pooled outcome data from previous systematic reviews in the general critical care population do not show a significant reduction in mortality with early tracheotomy, compared to late tracheostomy or prolonged intubation [7, 8, 49, 50]. However, two recent meta-analyses, including an updated review pooling results using the largest number of patients to date, did show significantly lower long-term mortality with early tracheostomy [6, 51]. We similarly found that performing an early tracheostomy in acutely brain-injured patients within the first 10 days of intubation might reduce long-term mortality and may also lower ICU mortality.
We also found that early tracheostomy significantly reduced the durations of mechanical ventilation and stay in the ICU, which may facilitate an earlier discharge to a non-ICU setting or transfer to a long-term acute care facility (LTACF) [52, 53]. A recent study found a steep rise over the past 20 years in rates of discharge to LTACFs after tracheostomy, from 40 % in 1993 to 72 % in 2012 [54]. Performing an early tracheostomy in acutely brain-injured patients may simply be shifting the location of deaths to these LTACFs, where overall mortality has been shown to be high [55]. However, this shift in discharge location may benefit the healthcare system by improving access to acute hospital resources, enabling discharge to a lower-intensity care setting at an earlier point in the admission period [46].
Strengths and Limitations
This review followed a predetermined protocol [56] for methodology and statistical analysis. Our extensive search strategy and inclusion of additional data from primary study authors allowed us to complete this novel synthesis of data for the role of early tracheostomy in brain-injured patients [6, 8, 49]. We incorporated unpublished literature, noting that such trials are of similar methodological quality compared to published trials [57] and did not find evidence of publication bias among the included trials.
Nevertheless, our study has a number of important limitations. First, the data are relatively sparse, and we were unable to incorporate data from four trials. Larger future trials are likely to modify the pooled analyses and conclusions. Secondly, studies were heterogeneous and included single and multicenter trials, percutaneous and surgical techniques, different timings of early tracheostomy (all within 10 days of intubation), and mixed acutely brain-injured populations. However, we did not identify any subgroup effects; and results were consistent in several a priori sensitivity analyses [10]. We were unable to explore in any depth subgroups of acutely brain-injured patients and cannot determine whether further trials should enroll homogenous patient groups by etiology, pathophysiological mechanisms or anatomic types [58], which may be relevant considerations for airway protection and ventilation failure.
Given our results, along with two recently terminated and unreported trials of early tracheostomy in severe brain injury [59, 60], physicians at the bedside lack definitive guidance regarding tracheostomy timing in brain-injured patients. Results from the ongoing SETPOINT 2 trial (ClinicalTrials.gov identifier: NCT02377167) may help to further define the role of early tracheostomy in at least one subgroup of severe stroke patients. In the meantime, clinical decisions for acutely brain-injured patients should be informed by existing RCTs or rigorously conducted meta-analyses of these RCTs [61, 62].
Conclusions
This systematic review suggests that performing an early tracheostomy in acutely brain-injured patients within the first 10 days of intubation may reduce long-term and ICU mortality while significantly reducing duration of mechanical ventilation and ICU stay. However, limited numbers of randomized patients and outcome events place serious limitations on the strength of these conclusions. Future trials of optimal timing of tracheostomy in this patient population should focus on patient-centered outcomes including comfort, mobility, functional outcomes, longer-term mortality, and discharge destination from the hospital.
References
Alali AS, Scales DC, Fowler RA, et al. Tracheostomy timing in traumatic brain injury: a propensity-matched cohort study. J Trauma Acute Care Surg. 2014;76(1):70–6 discussion 76-78.
Pinheiro Bdo V, Tostes Rde O, Brum CI, et al. Early versus late tracheostomy in patients with acute severe brain injury. J Bras Pneumol. 2010;36(1):84–91.
Gandia-Martinez F, Martinez-Gil I, Andaluz-Ojeda D, et al. Analysis of early tracheostomy and its impact on development of pneumonia, use of resources and mortality in neurocritically ill patients. Neurocirugia. 2010;21(3):211–21.
Wang HK, Lu K, Liliang PC, et al. The impact of tracheostomy timing in patients with severe head injury: an observational cohort study. Injury. 2012;43(9):1432–6.
Rizk EB, Patel AS, Stetter CM, et al. Impact of tracheostomy timing on outcome after severe head injury. Neurocrit Care. 2011;15(3):481–9.
Andriolo BN, Andriolo RB, Saconato H, et al. Early versus late tracheostomy for critically ill patients. The Cochrane database of systematic reviews 2015;1:Cd007271.
Szakmany T, Russell P, Wilkes AR, et al. Effect of early tracheostomy on resource utilization and clinical outcomes in critically ill patients: meta-analysis of randomized controlled trials. Br J Anaesth. 2015;114(3):396–405.
Siempos II, Ntaidou TK, Filippidis FT, et al. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(2):150–8.
Bouderka MA, Fakhir B, Bouaggad A, et al. Early tracheostomy versus prolonged endotracheal intubation in severe head injury. Journal of trauma. 2004;57(2):251–4.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken: Wiley; 2011.
Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE. 2013;8(12):e83138.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Haynes RB, McKibbon KA, Wilczynski NL, et al. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ. 2005;330(7501):1179.
Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc. 2006;94(1):41–7.
Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Barquist ES, Amortegui J, Hallal A, et al. Tracheostomy in ventilator dependent trauma patients: a prospective, randomized intention-to-treat study. Journal of Trauma—Injury, Infection and Critical Care. 2006;60(1):91–7.
Sugerman HJ, Wolfe L, Pasquale MD, et al. Multicenter, randomized, prospective trial of early tracheostomy. Journal of trauma. 1997;43(5):741–7.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1(3):247–62.
Koch T, Hecker B, Hecker A, et al. Early tracheostomy decreases ventilation time but has no impact on mortality of intensive care patients: a randomized study. Langenbecks Archives of Surgery. 2012;397(6):1001–8.
Rodriguez JL, Steinberg SM, Luchetti FA, et al. Early tracheostomy for primary airway management in the surgical critical care setting. Surgery. 1990;108(4):655–9.
Zheng Y, Sui F, Chen XK, et al. Early versus late percutaneous dilational tracheostomy in critically ill patients anticipated requiring prolonged mechanical ventilation. Chinese medical journal. 2012;125(11):1925–30.
Diaz-Prieto A, Mateu A, Gorriz M, et al. A randomized clinical trial for the timing of tracheotomy in critically ill patients: factors precluding inclusion in a single center study. Critical care (London, England) 2014;18(5):585.
Priyamvadha K, Rao S, Bundela Y, et al. Early versus late tracheostomy in critical brain injury: a prospective randomized study priyamvadha K, Rao S, Bundela Y, Gupta V, Dua S, Singh AK department of neurosciences, fortis hospitals, Noida, Delhi NCR, India. Brain Inj. 2012;26(4–5):504.
Rumbak MJ, Newton M, Truncale T, et al. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients.[Erratum appears in Crit Care Med. 2004 Dec; 32(12):2566]. Crit Care Med. 2004;32(8):1689–94.
Saffle JR, Morris SE, Edelman L. Early tracheostomy does not improve outcome in burn patients. J Burn Care Rehabilitation. 2002;23(6):431–8.
Trouillet JL, Luyt CE, Guiguet M, et al. Early percutaneous tracheotomy versus prolonged intubation of mechanically ventilated patients after cardiac surgery: a randomized trial.[Summary for patients in Ann Intern Med. 2011 Mar 15;154(6):I-38; PMID: 21403060]. Ann Intern Med. 2011;154(6):373–83.
El-Naggar M, Sadagopan S, Levine H, et al. Factors influencing choice between tracheostomy and prolonged translaryngeal intubation in acute respiratory failure: a prospective study. Anesth Analg. 1976;55(2):195–201.
Dunham CM, LaMonica C. Prolonged tracheal intubation in the trauma patient. J Trauma. 1984;24(2):120–4.
Sabouri Masih ETM, Hosseini Benham. The Effects of Early Tracheostomy on Outcomes of Patients with Severe Head Injury. Journal of Isfahan Medical School (IUMS) 2009;27(95):211-216.
Kiran Bylappa AM, Wilma Delphine Silvia CR, Dinesh Krishnamurthy, Mohammed Shabbir Pyarajan. A Comparative Study of Early and Late Tracheostomy in Patients Requiring Prolonged Tracheal Intubation. World Articles in Ear, Nose and Throat 2011;4(2). http://www.waent.org/archives/2011/Vol4-2/20111215-Tracheostomy-Intubation/late-tracheotomy.htm.
Young D, Harrison DA, Cuthbertson BH, et al. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. Jama. 2013;309(20):2121–9.
Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA—J Amer Med Association. 2010;303(15):1483–9.
Mohamed KAE, Mousa AY, ElSawy AS, et al. Early versus late percutaneous tracheostomy in critically ill adult mechanically ventilated patients. Egyptian J Chest Dis Tubercul. 2014;63(2):443–8.
Dunham CM, Cutrona AF, Gruber BS, et al. Early tracheostomy in severe traumatic brain injury: evidence for decreased mechanical ventilation and increased hospital mortality. Int J Burns Trauma. 2014;4(1):14–24.
Bösel J, Schiller P, Hook Y, et al. Stroke-related Early Tracheostomy versus Prolonged Orotracheal Intubation in Neurocritical Care Trial (SETPOINT): a randomized pilot trial. Stroke; a journal of cerebral circulation. 2013;44(1):21-28.
Blot F, Similowski T, Trouillet JL, et al. Early tracheotomy versus prolonged endotracheal intubation in unselected severely ill ICU patients. Intensive Care Med. 2008;34(10):1779–87.
Fayed AM, Elbadawy TH, Gamal MA, et al. Early gastrostomy and tracheostomy prevent ventilator associated pneumonia in traumatic brain injured patients. Intensive Care Med. 2012;38:S123.
Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–40.
Hospital-acquired pneumonia in adults. Diagnosis, assessment of severity initial antimicrobial therapy and preventive strategies a consensus statement American Thoracic Society November 1995. Amer J Resp Criti Care Med. 1996;153(5):1711–25.
Guidelines for the management of adults with hospital-acquired. ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
Griffiths J, Barber VS, Morgan L, et al. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330(7502):1243.
Scales DC. The implications of a tracheostomy for discharge destination. Am J Respir Crit Care Med. 2015;192(4):404–5.
Scales DC, Cuthbertson BH. Percutaneous dilatational tracheostomy: mostly safe, but do benefits outweigh risks? Criti care. 2014;18(2):117.
Simon M, Metschke M, Braune SA, et al. Death after percutaneous dilatational tracheostomy: a systematic review and analysis of risk factors. Criti care. 2013;17(5):258.
Huang H, Li Y, Ariani F, et al. Timing of tracheostomy in critically ill patients: a meta-analysis. PLoS ONE. 2014;9(3):e92981.
Meng L, Wang C, Li J, et al. Early vs late tracheostomy in critically ill patients: a systematic review and meta-analysis. Clin Respir J. 2015. 1–6. doi:10.1111/crj.12286.
Hosokawa K, Nishimura M, Egi M, et al. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Critical care. 2015;19:424.
Unroe M, Kahn JM, Carson SS, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153(3):167–75.
Arling G, Ofner S, Reeves MJ, et al. Care trajectories of veterans in the 12 Months after hospitalization for acute ischemic stroke. Circ Cardiovasc Qual Outcomes. 2015;8(6 Suppl 3):S131–40.
Mehta AB, Syeda SN, Bajpayee L, et al. Trends in tracheostomy for mechanically ventilated patients in the United States, 1993–2012. Am J Respir Crit Care Med. 2015;192(4):446–54.
Kahn JM, Benson NM, Appleby D, et al. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303(22):2253–9.
McCredie V, Alali A, Scales D, et al. Timing of tracheostomy in critically-ill acutely brain injured patients: a systematic review and meta-analysis, CRD42014010405. 2014 [cited 2015 December] http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014010405.
Hopewell S, McDonald S, Clarke M, et al. Grey literature in meta-analyses of randomized trials of health care interventions. Cochrane Database Syst Rev. 2007;(2):Mr000010.
Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–38.
Huttner HB. WEANING-Study: Weaning by Early Versus lAte Tracheostomy iN supratentorIal iNtracerebral Bleedings 2010 https://clinicaltrials.gov/ct2/show/NCT01176214.
Daumire R. A Prospective, Randomized Trial of Early Versus Late Tracheostomy in Trauma Patients With Severe Brain Injury 2006 https://clinicaltrials.gov/ct2/show/NCT00292097.
Inthout J, Ioannidis JP, Borm GF. Obtaining evidence by a single well-powered trial or several modestly powered trials. Statistical methods in medical research. 2016;25(2):538–52.
Cappelleri JC, Ioannidis JP, Schmid CH, et al. Large trials vs meta-analysis of smaller trials: how do their results compare? JAMA. 1996;276(16):1332–8.
Acknowledgments
We would like to thank Duncan Young (Adult Intensive Care Unit, John Radcliffe Hospital, University of Oxford, Oxford, England), François Blot (G. Nitenberg Intensive Care Unit, Gustave Roussy Institute, Villejuif, Paris, France), Julian Bösel (Department of Neurology, University of Heidelberg, Heidelberg, Germany), Agnes Laplanche (Service de Biostatistique et d’Epidemiologie, Gustave Roussy Institute, Villejuif, Paris, France), V. Marco Ranieri (Department of Anesthesia, University of Turin, Turin, Italy), and Akram M. Fayed (Department of Critical Care Medicine, Faculty of Medicine, University of Alexandria, Egypt) for generously providing us with additional subgroup information and clarification regarding their published trials.
Authors’ Contributions
V.A.M. contributed to the literature search, study design, data analysis, data interpretation, writing, critical revision, and final approval. A.A.A. contributed to the study design, data interpretation, critical revision, and final approval. N.K.J.A. contributed to the study design, data interpretation, critical revision, and final approval. D.C.S. contributed to data interpretation, critical revision, and final approval. G.D.R. contributed to the study design, data interpretation, critical revision, and final approval. B.H.C. contributed to the study design, data interpretation, critical revision, and final approval. A.B.N. contributed to the study design, data analysis, data interpretation, writing, critical revision, and final approval.
Funding
A.B.N. and this work were supported in part by the DeSouza Chair in Trauma Research. D.C.S. was supported by a Fellowship in Translational Health Research from the Physicians’ Services Incorporated Foundation. B.H.C. is supported by the University of Toronto, the Department of Anesthesia Merit Award. The opinions, results, and conclusions reported in this article are those of the authors. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare.
Additional information
Prospero
Our protocol was registered with PROSPERO (No: CRD42014010405).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McCredie, V.A., Alali, A.S., Scales, D.C. et al. Effect of Early Versus Late Tracheostomy or Prolonged Intubation in Critically Ill Patients with Acute Brain Injury: A Systematic Review and Meta-Analysis. Neurocrit Care 26, 14–25 (2017). https://doi.org/10.1007/s12028-016-0297-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0297-z