Abstract
Background
The efficacy of administering single bolus doses of 14.6 or 23.4 % hypertonic saline (HTS) to treat refractory intracranial hypertension has been demonstrated in the literature and has emerged as an important therapeutic option in treating these patients. However, many institutions lack experience with this therapy and there are few published studies evaluating the safety of repeated bolus dosing of HTS.
Methods
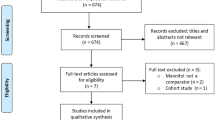
A retrospective review of patients admitted between January 2008 and July 2012 was conducted to evaluate the use of repeated dosing of HTS in patients with refractory intracranial hypertension. The primary objective was to evaluate the safety of repeated dosing of HTS assessed by documented adverse effects such as central pontine myelinolysis (CPM) and severe fluctuations in serum sodium concentrations. Secondary objectives were to evaluate the efficacy of repeated dosing HTS in reducing intracranial pressure (ICP) and to compare the dose–response relationship of 14.6 and 23.4 % doses.
Results
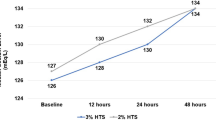
Fifty-five patients were included for evaluation, each receiving an average of 8.9 (range 2–61) doses of HTS. A statistically significant increase in mean serum sodium concentration occurred with the administration of HTS (p < 0.0001). No cases of CPM were identified. The use of HTS was found to be effective based on decreases in ICP after administration (p < 0.0001, mean ICP reduction: 10.1 mmHg, range 3–23.6 mmHg). The efficacy of 23.4 % saline in decreasing ICP was not found to be significantly different than 14.6 % saline (p = 0.23).
Conclusions
Repeat bolus dosing of 14.6 or 23.4 % HTS appears to be relatively safe and effective for treating refractory intracranial hypertension assuming there is frequent electrolyte monitoring and concomitant fluid management.


Similar content being viewed by others
References
Forsyth L, Liu-DeRyke X, Parker D, Rhoney D. Role of hypertonic saline for the management of intracranial hypertension after stroke and traumatic brain injury. Pharmacotherapy. 2008;28(4):469–84.
White H, Cook D, Venkatesh B. The use of hypertonic saline for treating intracranial hypertension after traumatic brain injury. Anesth Analg. 2006;102:1836–46.
Mortazavi M, Romeo A, Deep A, et al. Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis. J Neurosurg. 2012;116:210–20.
Tyagi R, Donaldson K, Loftus CM, Jallo J. Hypertonic saline: a clinical review. Neurosurg Rev. 2007;30:277–90.
Brain Trauma Foundation. Guidelines for the management of traumatic brain injury, 3rd edn. J Neurotrauma. 2007;24(supp 1):S1–106.
Diringer M, Zazulia A. Osmotic therapy: fact and fiction. Neurocrit Care. 2004;1(2):219–33.
Paredes-Andrade E, Solid C, Rockswold S, Odland R, Rockwold G. Hypertonic saline reduces intracranial hypertension in the presence of high serum and cerebrospinal fluid osmolalities. Neurocrit Care. 2012;17:204–10.
Ware M, Nemani V, Meeker M, Lee C, Morabito D, Manley G. Effects of 23.4 % sodium chloride solution in reducing intracranial pressure in patients with traumatic brain injury: a preliminary study. Neurosurgery. 2005;57:727–36.
Torre-Healy A, Marko N, Weil R. Hyperosmolar therapy for intracranial hypertension. Neurocrit Care. 2012;17(1):117–30.
Kerwin A, Schinco M, Tepas J, Renfro W, Vitarbo E, Muehlberger M. The use of 23.4 % hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma. 2009;67:277–82.
Qureshi A, Suarez J. Use of hypertonic saline solutions in treatment of cerebral edema and intracranial hypertension. Crit Care Med. 2000;28:3301–13.
Valentino A, Nau K, Miller D, Hanel R, Freeman W. Repeated dosing of 23.4 % hypertonic saline for refractory intracranial hypertension: a case report. J Vasc Interv Neurol. 2008;1(4):113–7.
Suarez J, Quereshi A, Bhardwaj A, et al. Treatment of refractory intracranial hypertension with 23.4 % saline. Crit Care Med. 1998;26(6):1118–22.
Lazaridis C, Neyen R, Bodle J, DeSantis S. High-osmolarity saline in neurocritical care: systematic review and meta-analysis. Crit Care Med. 2013;41:1353–60.
Eskandari R, Filtz M, Davis G, Hoesch R. Effective treatment of refractory intracranial hypertension after traumatic brain injury with repeated boluses of 14.6 % hypertonic saline. J Neurosurg. 2013;119:3338–46.
Koenig M, Bryan M, Lewin J 3rd, Mirski M, Geocadin R, Stevens R. Reversal of transtentorial herniation with hypertonic saline. Neurology. 2008;70:1023–9.
Rockswold G, Solid C, Paredes-Andrade E, Rockswold S, Jancik J, Quickel R. Hypertonic saline and its effects on intracranial pressure, cerebral perfusion pressure and brain tissue oxygen. Neurosurgery. 2009;65(6):1035–41.
Acknowledgments
The authors would like to acknowledge the contributions of the following colleagues: Paula Fuqua Pharm. D., CCRC (Department of Pharmacy, Mayo Clinic, Jacksonville, FL, USA) and Jamila Russeau Pharm. D., BCPS (Department of Pharmacy, Mayo Clinic, Jacksonville, FL, USA) for guidance and assistance in study protocol development and manuscript review. Colleen Thomas, MS (Department of Biostatistics, Mayo Clinic, Jacksonville, FL, USA) for general advice on using appropriate statistics for this study.
Conflict of interest
J. J. Lewandowski-Belfer, A. V. Patel, R. M. Darracott, D. A. Jackson, J. D. Nordeen, and W. D. Freeman declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lewandowski-Belfer, J.J., Patel, A.V., Darracott, R.M. et al. Safety and Efficacy of Repeated Doses of 14.6 or 23.4 % Hypertonic Saline for Refractory Intracranial Hypertension. Neurocrit Care 20, 436–442 (2014). https://doi.org/10.1007/s12028-013-9907-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-013-9907-1