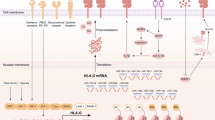
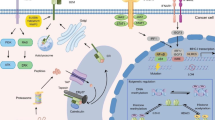
Abstract
Pancreatic cancer is one of the deadliest neoplasms, with a dismal 5-year survival rate of only 10%. The ability of pancreatic cancer cells to evade the immune system hinders an anti-tumor response and contributes to the poor survival rate. Downregulation of major histocompatibility complex (MHC) class I cell-surface expression can aid in immune evasion by preventing endogenous tumor antigens from being presented to cytotoxic T cells. Earlier studies suggested that epidermal growth factor receptor (EGFR) signaling can decrease MHC class I expression on certain cancer cell types. However, even though erlotinib (a tyrosine kinase inhibitor that targets EGFR) is an approved drug for advanced pancreatic cancer treatment, the impact of EGFR inhibition or stimulation on pancreatic cancer cell MHC class I surface expression has not previously been analyzed. In this current study, we discovered that EGFR affects MHC class I mRNA and protein expression by human pancreatic cancer cell lines. We demonstrated that cell-surface MHC class I expression is downregulated upon EGFR activation, and the MHC class I level at the surface is elevated following EGFR inhibition. Furthermore, we found that EGFR associates with MHC class I molecules. By defining a role in pancreatic cancer cells for activated EGFR in reducing MHC class I expression and by revealing that EGFR inhibitors can boost MHC class I expression, our work supports further investigation of combined usage of EGFR inhibitors with immunotherapies against pancreatic cancer.













Similar content being viewed by others
Change history
05 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12026-023-09442-9
References
Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10–27. https://doi.org/10.14740/wjon1166.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. Epub 2018/09/13. https://doi.org/10.3322/caac.21492.
Mundry CS, Eberle KC, Singh PK, Hollingsworth MA, Mehla K. Local and systemic immunosuppression in pancreatic cancer: targeting the stalwarts in the tumor’s arsenal. BBA - Reviews on Cancer. 2020;1874(1):188387. https://doi.org/10.1016/j.bbcan.2020.188387.
Emmrich J, Weber I, Nausch M, Sparmann G, Koch K, Seyfarth M, Lohr M, Liebe S. Immunohistochemical characterization of the pancreatic cellular infiltrate in normal pancreas, chronic pancreatitis and pancreatic carcinoma. Digestion. 1998;59(3):192–8. https://doi.org/10.1159/000007488
Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–31. https://doi.org/10.1097/00006676-200401000-00023
Campoli M, Chang C-C, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;20(Suppl 4):A40–5. https://doi.org/10.1016/s0264-410x(02)00386-9.
Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27(45):5869–85. https://doi.org/10.1038/onc.2008.273.
Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, Banh RS, Paulo JA, Wen KW, Debnath Y, Kim GE, Mancias JD, Fearson DT, Perera RM, Kimmelman AC. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100–5. https://doi.org/10.1038/s41586-020-2229-5.
Algarra I, Cabrera T, Garrido F. The HLA crossroad in tumor immunology. Hum Immunol. 2000;61(1):65–73.
Jiménez P, Cantón J, Collado A, Cabrera T, Serrano A, Real LM, García A, Ruiz-Cabello F, Garrido F. Chromosome loss is the most frequent mechanism contributing to HLA haplotype loss in human tumors. Int J Cancer. 1999;83(1):91–7.
Koopman LA, Corver WE, Van Der Slik AR, Giphart MJ, Fleuren GJ. Multiple genetic alterations cause frequent and heterogeneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J Exp Med. 2000;191(6):961–76.
Grandis JR, Falkner DM, Melhem MF, Gooding WE, Drenning SD, Morel PA. Human leukocyte antigen class I allelic and haplotype loss in squamous cell carcinoma of the head and neck: clinical and immunogenetic consequences. Clin Cancer Res. 2000;6(7):2794–802.
Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–73. https://doi.org/10.1146/annurev-immunol-032712-095910.
Ryschich E, Notzel T, Hinz U, Autschbach F, Ferguson J, Simon I, Weitz J, Frohlich B, Klar E, Buchler MW, Schmidt J. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res. 2005;11(2 Pt 1):498–504.
Concha-Benavente F, Srivastava RM, Ferrone S, Ferris RL. EGFR-mediated tumor immunoescape: the imbalance between phosphorylated STAT1 and phosphorylated STAT3. Oncoimmunology. 2013;2(12):e27215. https://doi.org/10.4161/onci.27215.
Leibowitz MS, Srivastava RM, Andrade Filho PA, Egloff AM, Wang L, Seethala RR, Ferrone S, Ferris RL. SHP2 is overexpressed and inhibits pSTAT1-mediated APM component expression, T-cell attracting chemokine secretion, and CTL recognition in head and neck cancer cells. Clin Cancer Res. 2013;19(4):798–808. https://doi.org/10.1158/1078-0432.CCR-12-1517.
Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, Mondello P, Han JE, Jarvis CA, Ulmert D, Xiang Q, Chang AY, Garippa RJ, Merghoub T, Wolchok JD, Rosen N, Lowe SW, Scheinberg DA. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol Res. 2016;4(11):936–47. https://doi.org/10.1158/2326-6066.CIR-16-0177.
Chen XH, Liu ZC, Zhang G, Wei W, Wang XX, Wang H, Ke HP, Zhang F, Wang HS, Cai SH, Du J. TGF-beta and EGF induced HLA-I downregulation is associated with epithelial-mesenchymal transition (EMT) through upregulation of snail in prostate cancer cells. Mol Immunol. 2015;65(1):34–42. https://doi.org/10.1016/j.molimm.2014.12.017.
Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16(12):649–56. https://doi.org/10.1016/j.tcb.2006.10.008.
Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12(1):3–20. https://doi.org/10.1002/1878-0261.12155.
Solassol I, Pinguet F, Quantin X. FDA- and EMA-approved tyrosine kinase inhibitors in advanced EGFR-mutated non-small cell lung cancer: safety, tolerability, plasma concentration monitoring, and management. Biomolecules. 2019;9(11):668. https://doi.org/10.3390/biom9110668.
Moore MJ, Goldstein D, Hamm J, Figer A, Hect JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotnib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. https://doi.org/10.1200/JCO.2006.07.9525.
Hao C, Li Z, Zhang X, Zhang H, Shang H, Bao J, Wang H. Expression and clinical significance of EGF and TGF-α in chronic pancreatitis and pancreatic cancer. Minerva Endocrinol. 2018;43(3):253–8. https://doi.org/10.23736/S0391-1977.17.02721-3.
Iwamura T, Katsuki T, Ide K. Establishment and characterization of a human pancreatic cancer cell line (SUIT-2) producing carcinoembryonic antigen and carbohydrate antigen 19–9. Jpn J Cancer Res. 1987;78(1):54–62.
Taniguchi S, Iwamura T, Katsuki T. Correlation between spontaneous metastatic potential and type I collagenolytic activity in a human pancreatic cancer cell line (SUIT-2) and sublines. Clin Exp Metastasis. 1992;10(4):259–66. https://doi.org/10.1007/BF00133561.
Tsutsumida H, Swanson BJ, Singh PK, Caffrey TC, Kitajima S, Goto M, Yonezawa S, Hollingsworth MA. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12(10):2976–87. https://doi.org/10.1158/1078-0432.CCR-05-1197.
Tan MH, Nowak NJ, Loor R, Ochi H, Sandberg AA, Lopez C, Pickren JW, Berjian R, Douglass HO Jr, Chu TM. Characterization of a new primary human pancreatic tumor line. Cancer Invest. 1986;4(1):15–23. https://doi.org/10.3109/07357908609039823.
Barton CM, Staddon SL, Hughes CM, Hall PA, O’Sullivan C, Kloppel G, Theis B, Russell RC, Neoptolemos J, Williamson RC, et al. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991;64(6):1076–82. https://doi.org/10.1038/bjc.1991.467.
Sun C, Yamato T, Furukawa T, Ohnishi Y, Kijima H, Horii A. Characterization of the mutations of the K-ras, p53, p16, and SMAD4 genes in 15 human pancreatic cancer cell lines. Oncol Rep. 2001;8(1):89–92. https://doi.org/10.3892/or.8.1.89.
Iguchi H, Morita R, Yasuda D, Takayanagi R, Ikeda Y, Takada Y, Shimazoe T, Nawata H, Kono A. Alterations of the p53 tumor-suppressor gene and ki-ras oncogene in human pancreatic cancer-derived cell-lines with different metastatic potential. Oncol Rep. 1994;1(6):1223–7. https://doi.org/10.3892/or.1.6.1223.
Okabe T, Yamaguchi N, Ohsawa N. Establishment and characterization of a carcinoembryonic antigen (CEA)-producing cell line from a human carcinoma of the exocrine pancreas. Cancer. 1983;51(4):662–8. https://doi.org/10.1002/1097-0142(19830215)51:4%3c662::aid-cncr2820510419%3e9.0.co;2-x.
Fogh J, Fogh JM, Orfeo T. One hudred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–6. https://doi.org/10.1093/jnci/59.1.221.
Torres MP, Rachagani S, Souchek JJ, Mallya K, Johansson SL, Batra SK. Novel pancreatic cancer cell lines derived from genetically engineered mouse models of spontaneous pancreatic adenocarcinoma: applications in diagnosis and therapy. PLoS ONE. 2013;8(11):e80580. https://doi.org/10.1371/journal.pone.0080580.
Scholtalbers J, Boegel S, Bukur T, Byl M, Goerges S, Sorn P, Loewer M, Sahin U, Castle JC. TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression. Genome Med. 2015;7:118. https://doi.org/10.1186/s13073-015-0240-5.
Bairoch A. The Cellosaurus, a cell line knowledge resource. J Biomol Tech. 2018;29(2):25–38.
Lee KM, Nguyen C, Ulrich AB, Pour PM, Ouellette MM. Immortalization with telomerase of the Nestin-positive cells of the human pancreas. Biochem Biophys Res Commun. 2003;301(4):1038–44. https://doi.org/10.1016/s0006-291x(03)00086-x.
Parham P, Brodsky FM. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981;3(4):277–99. https://doi.org/10.1016/0198-8859(81)90065-3.
Parham P, Barnstable CJ, Bodmer WF. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A, B. C antigens J Immunol. 1979;123(1):342–9.
Ladasky JJ, Shum BP, Canavez F, Seuanez HN, Parham P. Residue 3 of β2-microglobulin affects binding of class I MHC molecules by the W6/32 antibody. Immunogenetics. 1999;49(4):312–20. https://doi.org/10.1007/s002510050498.
Carreno BM, Hansen TH. Exogenous peptide ligand influences the expression and half-life of free HLA class I heavy chains ubiquitously detected at the cell surface. Eur J Immunol. 1994;24(6):1285–92. https://doi.org/10.1002/eji.1830240607.
Stam NJ, Vroom TM, Peters PJ, Pastoors EB, Ploegh HL. HLA-A- and HLA-B-specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin-fixed, paraffin-embedded tissue sections and in cryo-immuno-electron microscopy. Int Immunol. 1990;2(2):113–25. https://doi.org/10.1093/intimm/2.2.113.
Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35(3):177–88. https://doi.org/10.1016/s0161-5890(98)00026-1.
Brauswetter D, Gurbi B, Varga A, Varkondi E, Schwab R, Banhegyi G, Fabian O, Keri G, Valyi-Nagy I, Petak I. Molecular subtype specific efficacy of MEK inhibitors in pancreatic cancers. PLoS ONE. 2017;12(9):e0185687. https://doi.org/10.1371/journal.pone.0185687.
Spandidos A, Wang X, Wang H, Dragnev S, Thurber T, Seed B. A comprehensive collection of experimentally validated primers for polymerase chain reaction quantitation of murine transcript abundance. BMC Genomics. 2008;9:633. https://doi.org/10.1186/1471-2164-9-633.
Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38(Database issue):D792–9. https://doi.org/10.1093/nar/gkp1005
Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31(24):e154. https://doi.org/10.1093/nar/gng154.
Francavilla C, Papetti M, Rigbolt KT, Pedersen AK, Sigurdsson JO, Cazzamali G, Karemore G, Blagoev B, Olsen JV. Multilayered proteomics reveals molecular switches dictating ligand-dependent EGFR trafficking. Nat Struct Mol Biol. 2016;23(6):608–18. https://doi.org/10.1038/nsmb.3218.
Concha-Benavente F, Ferris RL. Reversing EGFR mediated immunoescape by targeted monoclonal antibody therapy. Front Pharmacol. 2017;8:332. https://doi.org/10.3389/fphar.2017.00332.
Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, Freeman GJ, Ferris RL. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76(5):1031–43. https://doi.org/10.1158/0008-5472.CAN-15-2001.
Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, Branstetter BFt, Ferrone S, Ferris RL. STAT1-induced HLA class I upregulation enhances immunogenicity and clinical response to anti-EGFR mAb Cetuximab therapy in HNC patients. Cancer Immunol Res. 2015;3(8):936–45. https://doi.org/10.1158/2326-6066.CIR-15-0053.
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21. https://doi.org/10.1158/0008-5472.CAN-14-0155.
Thibodeau S, Voutsadakis IA. FOLFIRINOX chemotherapy in metastatic pancreatic cancer: a systematic review and meta-analysis of retrospective and phase II studies. J Clin Med. 2018;7(1):7. https://doi.org/10.3390/jcm7010007.
Schlick K, Magnes T, Ratzinger L, Jaud B, Weiss L, Melchardt T, Greil R, Egle A. Novel models for prediction of benefit and toxicity with FOLFIRINOX treatment of pancreatic cancer using clinically available parameters. PLoS ONE. 2018;13(11):e0206688. https://doi.org/10.1371/journal.pone.0206688.
Chiorean EG, Cheung WY, Giordano G, Kim G, Al-Batran S-E. Real-world comparative effectiveness of nab-paclitaxel plus gemcitabine versus FOLFIRINOX in advanced pancreatic cancer: a systematic review. Ther Adv Med Oncol. 2019;11:1–17. https://doi.org/10.1177/1758835919850367.
Zhang B, Zhou F, Hong J, Ng DM, Yang T, Zhou X, Jin J, Zhou F, Cheng P, Xu Y. The role of FOLFIRINOX in metastatic pancreatic cancer: a meta-analysis. World J Surg Oncol. 2021;19(1):182. https://doi.org/10.1186/s12957-021-02291-6.
Krishnamoorthy M, Lenehan JG, Burton JP, Vareki SM. Immunomodulation in pancreatic cancer. Cancers. 2020;12:3340. https://doi.org/10.3390/cancers12113340.
Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I, Krshnamurthi S, Moravek C, O’Reilly EM, Philip PA, Ramanathan RK, Ruggiero JT, Shah MA, Urba S, Uronis HE, Lau MW, Laheru D. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(24):2545–56. https://doi.org/10.1200/JCO.2018.78.9636.
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang Y-J, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the Phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. https://doi.org/10.1200/JCO.19.02105.
Le DT, Picozzi VJ, Ko AH, Wainberg ZA, Kindler H, Wang-Gillam A, Oberstein P, Morse MA, Zeh HJ III, Weekes C, Reid T, Borazanci E, Crocenzi T, LoConte NK, Musher B, Laheru D, Murphy A, Whiting C, Nair N, Enstrom A, Ferber S, Brockstedt DG, Jaffee EM. Results from a Phase IIb, randomized, multicenter study of GVAX pancreas and CRS-207 compared with chemotherapy in adults with previously treated metastatic pancreatic adenocarcinoma (ECLIPSE Study). Clin Cancer Res. 2019;25:5493–502. https://doi.org/10.1158/1078-0432.CCR-18-2992.
Tsujikawa T, Crocenzi T, Durham JN, Sugar EA, Wu AA, Onners B, Nauroth JM, Anders RA, Fertig EJ, Laheru DA, Reiss K, Vonderheide RH, Ko AH, Tempero MA, Fisher GA, Considine M, Danilova L, Brockstedt DG, Coussens LM, Jaffee EM, Le DT. Evaluation of cyclophosphamide/GVAX pancreas followed by Listeria-mesothelin (CRS-207) with or without Nivolumab in patients with pancreatic cancer. Clin Cancer Res. 2020;26(14):3578–88. https://doi.org/10.1158/1078-0432.CCR-19-3978.
Zheng L, Ding D, Edil BH, Judkins C, Durham JN, Thomas DL II, Bever KM, Mo G, Solt SE, Hoare JA, Bhattacharya R, Zhu Q, Osipov A, Onner B, Purtell KA, Cai H, Parkinson R, Hacker-Prietz A, Herman JM, Le DT, Azad NS, De Jesus-Acosta AMC, Blair AB, Kim V, Soares KC, Manos L, Cameron JL, Makary MA, Weiss MJ, Schulick RD, He J, Wolfgang C, Thompson ED, Anders RA, Sugar E, Jaffee EM, Laheru DA. Vaccine-induced intratumoral lymphoid aggregates correlate with survival following treatment with a neoadjuvant and adjuvant vaccine in patients with resectable pancreatic adenocarcinoma. Clin Cancer Res. 2021;27:1278–86. https://doi.org/10.1158/1078-0432.CCR-20-2974.
Hammel P, Huguet F, vanLaethem JL, Goldstein D, Glimelius B, Artru P, Borbath I, Bouche O, Shannon J, Andre T, Mineur L, Chibaudel B, Bonnetain F, Louvet C. Group LAP07 Trial Group. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–53. https://doi.org/10.1001/jama.2016.4324.
Lizotte PH, Hong RL, Luster TA, Cavanaugh ME, Taus LJ, Wang S, Dhaneshwar A, Mayman N, Yang A, Kulkarni M, Badalucco L, Fitzpatrick E, Kao HF, Kuraguchi M, Bittinger M, Kirschmeier PT, Gray NS, Barbie DA, Janne PA. A high-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic T-lymphocyte tumor cell killing. Cancer Immunol Res. 2018;6(12):1511–23. https://doi.org/10.1158/2326-6066.CIR-18-0193.
Chan YH. Biostatistics 104: correlational analysis. Singapore Med J. 2003;44(12):614–9.
Akoglu H. User’s guide to correlation coefficients. TurkJ Emerg Med. 2018;18(3):91–3. https://doi.org/10.1016/j.tjem.2018.08.001.
Girdlestone J, Milstein C. Differential expression and interferon response of HLA class I genes in thymocyte lines and response variants. Eur J Immunol. 1988;18(1):139–43. https://doi.org/10.1002/eji.1830180121.
Johnson DR. Locus-specific and constitutive and cytokine-induced HLA class I gene expression. J Immunol. 2003;170(4):1894–902. https://doi.org/10.4049/jimmunol.170.4.1894.
Girdlestone J, Isamat M, Gewert D, Milstein C. Trancriptional regulation of HLA-A and -B: differential binding of members of the Rel and IRF families of transcription factors. Proc Natl Acad Sci USA. 1993;90(24):11568–72. https://doi.org/10.1073/pnas.90.24.11568.
Jongsma MLM, Guarda G, Spaapen RM. The regulatory network behind MHC class I expression. Mol Immunol. 2019;113:16–21. https://doi.org/10.1016/j.molimm.2017.12.005.
Chappell PE, Meziane EK, Harrison M, Magiera L, Hermann C, Mears L, Wrobel AG, Durant C, Nielsen LL, Buus S, Ternette N, Mwangi W, Butter C, Nair V, Ahyee T, Duggleby R, Madrigal A, Roversi P, Lea SM, Kaufman J. Expression levels of MHC class I molecules are inversely correlated with promiscuity of peptide binding. Elife. 2015;4:e05345. https://doi.org/10.7554/eLife.05345.
Myers NB, Wormstall E-M, Hansen TH. Differences among various class I molecules in competition for β2m in vivo. Immunogenetics. 1996;43(6):384–7. https://doi.org/10.1007/BF02199807.
Joyce S. Traffic control of completely assembled MHC class I molecules beyond the endoplasmic reticulum. J Mol Biol. 1997;266(5):993–1001. https://doi.org/10.1006/jmbi.1996.0822.
Weidanz JA, Nguyen T, Woodburn T, Neethling FA, Chiriva-Internati M, Hildebrand WH, Lustgarten J. Levels of specific peptide-HLA class I complex predicts tumor cell susceptibility to CTL killing. J Immunol. 2006;177(8):5088–97. https://doi.org/10.4049/jimmunol.177.8.5088.
Rivoltini L, Barracchini KC, Biggiano V, Kawakami Y, Smith A, Mixon A, Restifo NP, Topalian SL, Simonis TB, Rosenberg SA, Marincola FM. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res. 1995;55:3149–57.
Funding
This work was supported by the National Institutes of Health (R21 CA223429, P30 CA036727, T32 CA009476, U54 GM115458, P50 CA127297), the State of Nebraska, and a Graduate Studies Office Fellowship. The University of Nebraska Medical Center (UNMC) Flow Cytometry Research Facility is supported through the Office of the Vice Chancellor for Research, the Nebraska Research Initiative, the Fred and Pamela Buffett Cancer Center, the University of Nebraska Foundation, the Nebraska Bankers Fund, and the National Institutes of Health Shared Instrument Grant Program. Assistance was also provided for this project by the UNMC Molecular Diagnostics Histocompatibility Laboratory.
Author information
Authors and Affiliations
Contributions
Shelby Knoche and Joyce Solheim contributed to the design of the study. Shelby Knoche performed experiments and bioinformatics analysis with assistance from Alaina Larson and Gabrielle Brumfield, and Shelby Knoche and Joyce Solheim completed the analysis of the data. Steven Cate and William Hildebrand provided HLA class I typing of the S2-013 cell line. The manuscript was drafted by Shelby Knoche and edited by Alaina Larson, Gabrielle Brumfield, Steven Cate, William Hildebrand, and Joyce Solheim. All authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Knoche, S.M., Larson, A.C., Brumfield, G.L. et al. Major histocompatibility complex class I molecule expression by pancreatic cancer cells is regulated by activation and inhibition of the epidermal growth factor receptor. Immunol Res 70, 371–391 (2022). https://doi.org/10.1007/s12026-022-09262-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-022-09262-3