Abstract
Immune checkpoint blockades have been prized in circumventing and ablating the impediments posed by immunosuppressive receptors, reaching an exciting juncture to be an innovator in anticancer therapy beyond traditional therapeutics. Thus far, approved immune checkpoint blockades have principally targeted PD-1/PD-L1 and CTLA-4 with exciting success in a plethora of tumors and yet are still trapped in dilemmas of limited response rates and adverse effects. Hence, unveiling new immunotherapeutic targets has aroused immense scientific interest in the hope of expanding the clinical application of immune checkpoint blockades to scale new heights. Human leukocyte antigen-G (HLA-G), a non-classical major histocompatibility complex (MHC) class I molecule, is enriched on various malignant cells and is involved in the hindrance of immune effector cells and the facilitation of immunosuppressive cells. HLA-G stands out as a crucial next-generation immune checkpoint showing great promise for the benefit of cancer patients. Here, we provide an overview of the current understanding of the expression pattern and immunological functions of HLA-G, as well as its interaction with well-characterized immune checkpoints. Since HLA-G can be shed from the cell surface or released by various cells as free soluble HLA-G (sHLA-G) or as part of extracellular vesicles (EVs), namely HLA-G-bearing EVs (HLA-GEV), we discuss the potential of sHLA-G and HLA-GEV as predictive biomarkers. This review also addresses the advancement of HLA-G-based therapies in preclinical and clinical settings, with a focus on their clinical application in cancer.
Similar content being viewed by others
Background
In the process of oncogenesis and development, malignant tumor cells evolve constantly and strive hard to build strong defenses against the immune system [1]. Tumor cells take full advantage of immune checkpoint molecules to restrain the function of immune cells, protecting themselves from attacks and achieving immune escape. On the contrary, a growing body of evidence demonstrates that strategies targeting immune checkpoints exhibit the potential to restore or enhance the anti-tumor capacity [2], leading to the emergence of immune checkpoint blockade (ICB) therapy. To date, several immune checkpoint targeting agents have gained approvals for the treatment of multiple cancers as monotherapy or in combination with other therapeutics [3,4,5,6]. With the rise of ICB in immuno-oncology, the treatment of oncology has also entered the era of immunotherapy. Despite the unprecedented breakthrough achieved by ICB therapies, considerable challenges persist, namely the limited response rate, severe hazardous adverse events, and acquired resistance [7]. More than half of these patients are likely to experience immune-related adverse events (irAEs), with 0.3 ~ 1.3% of the cases being fatal, leading to treatment interruption and poor outcomes [8]. Therefore, identification of novel immune checkpoints and formulation of innovative strategies are urgently needed, thereby broadening the clinical application spectrum of ICB therapies.
Human leukocyte antigen-G (HLA-G), a non-classical major histocompatibility complex (MHC) class I molecule, was demonstrated to be integral to maternal–fetal tolerance initially and deemed to be a vital immune checkpoint. HLA-G engages with ILT2/4 and KIR2DL4 to elicit the suppression of immune effector cells, including cytotoxic T cells and natural killer (NK) cells, as well as the expansion of immunosuppressive cells like Treg cells and myeloid-derived suppressor cells (MDSCs), resulting in an immunosuppressive microenvironment and contributing to the immune escape of tumor cells [9]. Due to the promising potential of HLA-G blockade, ongoing researches about why and how HLA-G-based therapeutic approaches quell the malignant phenotype are increasing, as exemplified by the deciphering of the roles of HLA-G positive cancer cells in colorectal cancer using spatial and single-cell transcriptomics [10], and the employment of HLA-G-targeted CAR-T cells for the direct killing of EGFR-mutated and overexpressed oral cancers [11]. In addition, several clinical trials have been designed to evaluate the effectiveness of HLA-G inhibitors as monotherapy or in combination therapy for the treatment of solid tumors. Therefore, HLA-G-based therapeutic approaches are on the way to become a hot spot in the field of tumor immunotherapy.
In this review, we overview the fundamentals and immunobiology of HLA-G and pinpoint its discrepancy with PD-L1. In addition, we highlight the latest preclinical and clinical trial advances, with the aim of providing further insight into HLA-G-based modalities for cancer treatment and proposing new perspectives on targeted therapy for cancer patients.
Molecular structure and distribution characteristics of HLA-G
The HLA-G gene, being highly homologous to the classical MHC molecules, is located in chromosome 6p21.3 containing eight exons encoding the signal peptide, the extracellular α1-α3 domain, the transmembrane domain, and the intracellular region, successively. The second codon of exon 6 acts as an early stop codon and the resulting intracellular cytoplasmic tail of HLA-G is shorter compared to the classical MHC molecules [9]. The HLA-G gene also comprises seven introns. Moreover, it has been well-established that at least seven different isoforms were generated via alternative splicing. Isoforms HLA-G1, HLA-G2, HLA-G3, and HLA-G4 retain the transmembrane domain to express the corresponding membrane-bound proteins, whereas the HLA-G5, HLA-G6, and HLA-G7 exist in soluble form due to the absence of transmembrane domain [12,13,14]. The characteristics of each isoform are summarized in Table 1.
Unlike classical MHC class I molecules, the expression of HLA-G is highly restricted. In physiological conditions, HLA-G is exclusively found in a few tissues and cells, including extravillous trophoblasts of the placenta [5], cornea [6], and thymus epithelial cell [7], contributing to immune tolerance through interaction with corresponding ligands on immune cells. However, overexpression of HLA-G is induced under multiple pathological conditions, including malignant tumors, viral or microbial infections [8,9,10,11], and autoimmune diseases [12, 13] Convergent data implicated that HLA-G was enriched in several tumors, thereby fueling the immune evasion.
Underlying mechanisms regulating HLA-G expression
Several mechanisms are involved in the comprehensive modulation of HLA-G, namely epigenetic regulation, transcription factors, post-transcriptional regulation, and post-translational modification, as presented in Fig. 1.
-
1.
Epigenetic regulation
Regulation of HLA-G expression. The transcriptional activity of HLA-G is modulated by epigenetic alterations and several transcription factors in response to environmental and drug stimuli. A number of miRNAs can directly target the 3'UTR of HLA-G mRNA and impede HLA-G translation. HLA-G is also subjected to diverse post-translational modifications, including ubiquitination, nitration, and glycosylation. Moreover, various hormones, cytokines, and chemotherapy drugs can enhance HLA-G expression via direct binding with HLA-G gene or activation of downstream signaling pathways. Ac acetylation, Me methylation, Ub ubiquitination, Glc glycosylation, NO nitration
Covalent binding of methyl group (-CH3) to cytosine residues could promote methylation of CpG islands in the HLA-G promoter region, silencing the transcription of HLA-G gene [15]. In this context, expression of DNA methyltransferases (DNMTs), a contributing factor to hypermethylation of promotor, was inversely correlated with HLA-G protein level [16]. On the contrary, DNMT inhibitor 5-Aza could induce a rise in HLA-G mRNA and protein in breast cancer cells [17], which was also confirmed in renal cell carcinoma, melanoma, pancreatic cancer, ovarian cancer, glioblastoma and choriocarcinoma [18,19,20,21,22]. In addition, histone acetylation was augmented in the presence of histone deacetylase inhibitor (HDACi) sodium butyrate, valproic acid, or trichostatin A (TSA), reinvigorating the transcription of HLA-G [19, 23]. Indeed, as one of the important intrinsic drivers of gene regulation, epigenetics could be widely exploited in the field of cancer treatment. Given that inhibitors of epigenetic regulators could potentiate HLA-G expression, their combination with HLA-G blockade deserves further exploration as novel therapeutics.
-
2. Transcriptional regulation
In addition to epigenetic mechanisms, transcriptional regulation mediated by multiple regulatory elements is involved in the regulation of HLA-G expression. It should be noted that the promoter of HLA-G is extremely divergent from that of any other MHC class I gene. The promoter regions of MHC class I genes contain regulatory modules that activate transcription, mainly including enhancer A, the interferon-stimulated responsive element (ISRE), and the SXY module. However, in HLA-G promoter, the enhancer A and SXY modules are modified, while the ISRE is absent. HLA-G exhibited a strong binding affinity to the p50 homodimeric subunit of NF-κB due to the sequence differences in the enhancer A (κB1 and κB2 sites), whereas none of the other NF-κB subunits with transcriptional properties, including p65 and c-Rel, recognized these sites [24]. Moreover, the MHC master transcription factors NLRC5 and CIITA failed to bind to the HLA-G gene promoter owing to the absence of the conserved SXY module [25]. As early as 2002, transcriptional regulators C-jun, cAMP-responsive element binding protein (CREB)-1, and ATF1 have been proven to engage with the three CRE/TRE regulatory elements in the HLA-G promoter region, potentiating the basal level of HLA-G promoter activity through trans-activation [26]. Consistently, Michael Friedrich et al. have recently validated the binding of CREB to HLA-G promotor sequences in renal cancer cells [17]. In addition, the binding of IRF-1, HIF-1, or HSF-1 to corresponding elements augmented HLA-G transcription in response to external stimuli, respectively [27,28,29]. Unlike HLA-E and HLA-F, HLA-G was barely responsive to the regulation of RFX5, due to the lack of transcriptional scaffold created by RFX5, resulting in the inability of NLRC5 and CIITA to bind to the promoter [30]. Given its unique transcriptional regulation pattern, it is reasonable to conclude that HLA-G functions distinctively throughout the immune response. Conversely, the long interspersed nuclear elements (LINE)-1 scattered throughout the upstream region of HLA-G leading to the silencing of the gene [31], whereas the binding of Ras responsive element binding protein (RREB)-1 to Ras responsive element was capable of restraining HLA-G transcription [32], which might partially account for the restricted expression of HLA-G under physiological conditions. Overall, novel transcription factors of HLA-G remain to be explored in order to screen attractive candidate drugs that might expand the applicability of HLA-G-based therapeutics.
-
3.
Post-transcriptional regulation
MicroRNAs (miRNAs) are a class of highly conserved endogenous non-coding single-stranded RNAs with a total length of approximately 22 nt, modulating the transcription of target genes via base-pair complementation [33]. Indeed, a number of miRNAs could interact with the 3'UTR region to hinder the translation of HLA-G, including miR-148a, miR-148b, miR-152, miR-133a, miR-139-3p, miR-548, miR-608, miR-628, miR-138-1-3p, miR19a, miR-19b-1, miR744 [34,35,36,37,38,39,40,41,42]. The varying levels of HLA-G expression within the tissues might be attributed to the presence of different types and amounts of miRNAs. Placental tissues exhibited relatively low expression of miR-148a and miR-152, making the placenta one of the few tissues rich in HLA-G [38]. Conversely, the high expression of miR-148a and miR-152 has been demonstrated to be related to the reduced HLA-G protein level in renal cancer and colon cancer [34, 43]. Notably, miRNAs differed in affinity for 3'UTR sequences in HLA-G, as denoted by the fact that miR-152, miR148a, miR-148b, and miR-133a had sequentially weaker affinity for HLA-G validated by miTRAP technology [34]. In addition, miR-139-3p could bind to the non-polymorphic sequences in HLA-G 3'UTR region specifically, while miR-608 exclusively bound to the polymorphic sequences [37]. Convincing data is required to elucidate the underlying mechanisms of miRNAs regulation and their correlation with cancers.
-
4.
Post-translational modification
Protein expression and function could be modified by the addition or removal of specific chemical groups to amino acids, namely the post-translational modifications, such as phosphorylation, acetylation, ubiquitination, and glycosylation, which together constitute a complex network of regulation. Ubiquitinated HLA-G complexes have been found in the circulation and unleashed as exosomes, favoring tumor spread [44]. The detection of HLA-G nitration in patient plasma and exudate might be associated with the metalloproteinase-mediated shedding of HLA-G from cell surface, and that nitrated HLA-G retained its original biological function and effectively inhibited NK cytotoxicity [45, 46]. HLA-G in body fluids could be used as a humoral biomarker for several diseases, especially malignancies. Thus, ascertaining whether the measurement of ubiquitinated or nitrated HLA-G would achieve better specificity and sensitivity will be crucial, offering predictive and therapeutic opportunities. Moreover, glycosylated HLA-G was produced by placental trophoblast cells and secreted into the amniotic fluid, being crucial for the maintenance of maternal–fetal immune tolerance [47], but mechanistic evidence for the role of HLA-G in tumor immune-evasive is still missing.
-
5.
Others
In addition, hormones and cytokines have also been reported to modulate HLA-G expression. Progesterone could directly bind to the progesterone response element (PRE) in the HLA-G promoter and induce HLA-G transcription in trophoblast cells, breast cancer cells, and choriocarcinoma cells [48,49,50]. HLA-G protein level was elevated in breast cancer cells in response to estradiol administration [51]. Likewise, prolactin exhibited the same promoting effect on trophoblast cells [52]. The low expression of HLA-G in trophoblast cells has been validated to be a contributing factor to recurrent miscarriage, whereas glucocorticoids could potently reverse the phenomenon and enhance HLA-G expression in a concentration-dependent manner, hinting that glucocorticoids might be beneficial to protecting the fetus from immune system attack in patients with recurrent miscarriage [53]. Moreover, mounting studies have shown that epidermal growth factor (EGF), interferons (IFN-α, IFN-β, IFN-γ), interleukins (IL-1β, IL-2, IL-4, IL-10), transforming growth factor (TGF-β) and tumor necrosis factor (TNF-α) could regulate HLA-G expression [52, 54,55,56,57,58,59,60,61,62,63,64]. It is noteworthy that in certain HLA-G-negative cell lines, IFN-γ failed to upregulate HLA-G levels but could further amplify 5-Aza-induced HLA-G expression, hinting an important role of IFN-γ in maintaining HLA-G expression. Notably, IFN-γ failed to upregulate HLA-G levels in some HLA-G-negative cell lines [65, 66].
Studies on HLA-G expression being modulated by aberrant signaling pathways are limited. Chemotherapy upregulated HLA-G by inhibiting DNMT1 and inducing demethylation of TAP1, providing opportunities for anti-HLA-G CAR-NK cells to eliminate cancer cells [22]. More recent research demonstrated that EGFR promoted IL-1β secretion via NLRP3 inflammasome to induce HLA-G expression, resulting in immunosuppression in oral squamous cell carcinoma (OSCC) [11].
HLA-G expression and clinical significance in cancer
HLA-G is engaged in the evolution of normal tissue cells and their transformation into malignant cells, and its expression and polymorphisms have been linked to precancerous lesions and cancer susceptibility. Long-term and persistent infection with high-risk HPV viruses would result in cervical intraepithelial neoplasia (CIN), approximately 30–40% of which will progress to invasive cervical cancer [67, 68]. Growing studies have suggested that HLA-G 3'UTR polymorphisms are likely to be independent risk factors for HPV infection, as indicated by the intimate association between HLA-G 14bp In/ + 3142G/ + 3142C allele and susceptibility to HPV and the progression of cervical lesions [69]. It has also been shown that amplification of HLA-G + 3142C allele was a contributing factor to sHLA-G upregulation and significantly correlated with susceptibility to HPV [70,71,72,73]. In addition, HLA-G*01:01:02/01:01:08 alleles and HLA-G*01:04:01 homozygote were associated with increased risk and decreased risk of infection, respectively [74, 75]. HLA-G*01:01:02 and HLA-G*01:03 alleles were found to be significantly related to persistent HPV16 infection [75]. HLA-G has been implicated in malignant transformation [76], increasing throughout CIN progression [77,78,79] and peaking in cervical cancer [79,80,81]. Along similar lines, in hepatocellular carcinoma, the HLA-G 14bp I/D polymorphism was significantly correlated with tumor development in HBV/HCV( +) cases rather than in the HBV/HCV(-) subset [82]. A large-scale GWAS study identified HLA-G as a principal locus related to the risk of CRC [83], with DelG haplotype and InsC haplotype indicating an increased or decreased risk of developing CRC, respectively [84]. In breast cancer, HLA-G + 3142G was a protective factor for cancer susceptibility, while the + 3142C allele acted in the opposite way [85]. 14bp Del and + 3010/ + 3142/ + 3187 variants were found to be of great value in distinguishing breast cancer patients from the general population [86]. Similarly, HLA-G 14bp Del allele and 14bp Del/ + 3142 C variants displayed a higher frequency in patients with gastric cancer [87]. Thus, HLA-G polymorphic variants are closely related to the development or progression of several malignant pathologies.
HLA-G was aberrantly enriched in a variety of tumors (Fig. 2), including colorectal cancer [88], gastric cancer [89], ovarian cancer [90,91,92,93], thyroid cancer [40], cervical cancer [78, 94], and endometrial cancer [95,96,97]. In colorectal cancer, HLA-G was observed to aggregate at the invasion front in cancer foci, which was conducive to the formation of an immunosuppressive microenvironment, favoring further invasion and metastasis [10]. It is desirable to explore whether this phenomenon is universal in pan-cancer. HLA-G exhibited a strong co-localization with CA125 in ovarian cancer [98], indicating that HLA-G might be a valuable predictive biomarker for early ovarian cancer. HLA-G expression has been reported to be modulated by the grading and staging of the tumor, and HLA-G protein levels were significantly higher in patients with advanced stages in comparison to early-stage and normal [11, 98,99,100,101,102]. Inconsistently, HLA-G expression did not correlate with disease stage in thyroid cancer [103]. HLA-G is suggestive of tumor progression and recurrence as denoted by the positive correlation between HLA-G high-expression and advanced stage [99, 104,105,106,107], distant metastasis [108], lymph node metastasis [102, 105, 109], and higher recurrence rates [90, 101, 110]. In ovarian cancer, compared to the tumor cells in situ, metastatic tumor cells in ascites had higher expression of HLA-G, which was inversely correlated to the frequency of immune cell infiltration and positively related to disease progression, tumor metastasis, and poor prognosis [93, 98]. Also, extensive evidence has confirmed that high HLA-G expression was positively related to shorter survival [43, 64, 88, 90, 102, 111,112,113,114,115,116,117,118,119,120] and was an independent risk factor for prognosis [51, 115, 121,122,123,124,125,126]. Recently, an eight-gene prognostic model constructed on the basis of HLA-G-driven differential genes demonstrated good predictive value for the prognosis of cervical cancer patients (AUC = 0.896) [127]. HLA-G may also function as a biomarker for screening potential benefit populations and evaluating therapeutic efficacy, as exemplified by a substantial reduction of HLA-G expression after chemotherapy administration, which probably arises from the increased sensitivity to chemotherapy of HLA-G+ tumor cells [128], and a higher response rate to chemotherapy in ovarian cancer patients with high HLA-G expression compared to the counterparts with low HLA-G expression [129]. Similarly, HLA-G receptor ILT2 was related to worse adjuvant chemotherapy responses and indicative of poor response to anti-PD-1/PD-L1 therapies when combined with the absence of CD8+T cells [130]. In colorectal cancer, HLA-G allele could be used to screen patients who might show a good response to first-line FOLFIRI chemotherapy regimens, as the response rate compared favorably in patients carrying + 3010G and + 3187G allele with that seen in patients carrying + 2960-Ins allele [131]. Collectively, the above results indicate that HLA-G has potential clinical implications in guiding the treatment of cancer patients, and may serve as a potential diagnostic and prognostic biomarker.
Intriguingly, sHLA-G in circulation has fueled the tumor cells to spread immunosuppressive signals. Elevated levels of sHLA-G have been observed in gastric cancer, ovarian cancer, cervical cancer, and endometrial cancer, hinting at the potential of sHLA-G in distinguishing malignant from benign and defining tumor stages [82, 91, 132,133,134,135,136,137]. Besides, serum sHLA-G levels exhibited remarkable diagnostic value for thyroid cancer (AUC = 0.925), which was linked to clinicopathological features including tumor size, lymph node metastasis, and degree of differentiation, and showed significant negative correlation with 1 year and 3 year survival rates [138]. In colorectal cancer, sHLA-G levels in plasma were positively correlated with the number of metastatic sites [139, 140] and inversely related to survival [108], whereas high levels of sHLA-G in saliva were suggestive of later disease stage [141]. High levels of plasma sHLA-G were found in patients with locally advanced breast cancer [142], rather than in the triple-negative breast cancer (TNBC) subgroup [143, 144]. This difference may be attributed to the lack of hormone receptors, as suggested by experimental studies that have shown elevated levels of sHLA-G in hormone-receptor-positive patients compared to their negative counterparts [51]. A recent study revealed that in TNBC patients, sHLA-G plasma levels were significantly increased after neo-adjuvant and adjuvant chemotherapy compared with pre-treatment, and were associated with distant metastases and poorer disease outcomes [144]. In contrast, plasma sHLA-G dramatically declined within 30 days after radical hysterectomy [145]. Hence, whether monitoring the dynamic changes of sHLA-G in plasma could be employed in evaluating efficacy and predicting recurrence in tumor patients after surgery or chemotherapy warrants further investigation. Moreover, increased amount of HLA-G-bearing extracellular vesicles (HLA-GEV) predisposed epithelial ovarian cancer patients to disease progression and poor prognosis, being indicative of higher disease risks [146]. Recently, HLA-G protein expression on the membrane of exosomes has also been confirmed in gastric cancer [136]. Compared with tissue biopsies for intra-tumor HLA-G expression, liquid biopsies for analysis of HLA-GEV are easy in operation, cost-effective, and noninvasive. It is reasonable to speculate that the measurement of HLA-GEV in combination with sHLA-G might be a supplement to tumor markers in cancer screening.
In conclusion, owing to the heterogeneity of the tumors and the discrepancies in detection tools and reagents, studies regarding HLA-G expression in cancers have shown mixed results (Table 2). Therefore, it is imperative to conduct additional pre-clinical and clinical research to pinpoint the role of HLA-G in precancerous and malignant lesions. This will help advance the potential of HLA-G as a predictive biomarker for early cancers from bench to bedside.
HLA-G interplay with immune receptors
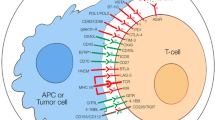
Several receptors for HLA-G have been identified, including ILT2, ILT4, and KIR2DL4. ILT-2 and ILT-4 are the members of the leukocyte immunoglobulin-like receptor (LILR) family. Each consists of four extracellular Ig-like domains, with D1D2 responsible for HLA-G binding and D3D4 acting as a scaffold [147]. ILT2 and ILT4 both have a cytoplasmic tail that contains immunoreceptor tyrosine-based inhibitory motifs (ITIM) that interact with tyrosine phosphatases (SHP1/SHP2) and initiate inhibitory signaling [148]. It has been revealed that the ILTs have a broad specificity for MHC class I molecules, but they exhibit the highest affinity for HLA-G [149]. Moreover, the binding affinity of HLA-G to ILTs was improved by the dimerization of HLA-G due to the increased exposure of binding site [147, 150]. ILT2 is expressed on various immune cells, including T cells, B cells, NK cells, myeloid-derived suppressive cells (MDSCs), dendritic cells (DCs), and monocytes/macrophages, whereas ILT4 is exclusively presented on DCs, monocytes/macrophages, neutrophils and MDSCs [151,152,153,154,155]. KIR2DL4 belongs to the killer cell immunoglobulin (Ig) like receptor (KIR) family, with a charged arginine residue near the top of its transmembrane region that can associate with the immunoreceptor tyrosine-based activating motif (ITAM). It also has a single intracellular ITIM domain and exhibits weak inhibitory potential [156]. Due to its unique structure, KIR2DL4 functions as both an activating and inhibitory receptor. KIR2DL4 lacks a D1 domain, and its interaction with HLA-G is mediated by the D0 or D2 domain [157]. In contrast to ILT2/4, which is expressed in a wide range of cells, KIR2DL4 is predominantly found on NK cells [156]. A recent study demonstrated that KIR2DL4 synergized with FcRγ to augment NK cell activation and degranulation, whereas the interaction of HLA-G with KIR2DL4 attenuated NK cell cytotoxicity in HER2-positive breast cancer [158]. Other receptors, such as CD160, are expressed by endothelial cells, and the interaction between HLA-G and CD160 can lead to apoptosis and thus inhibit angiogenesis [159]. In addition, it has been reported that HLA-G binding to CD8 modulates the activation and apoptosis of cytotoxic T cells and NK cells [160,161,162].
HLA-G functions on immune cells
Broad evidence has suggested that HLA-G could modulate innate and adaptive immunity through its interaction with ILT2/4 and KIR2DL4 (Fig. 3). HLA-G restrained CD4+ and CD8+ T cells proliferation [163, 164], and concomitantly induced apoptosis through Fas/FasL signaling pathway via CD8 ligation [160, 162]. Furthermore, HLA-G fostered the differentiation of the CD4 + T cells into Treg cells [165, 166], while HLA-G1-induced CD4+ T cells would enter a long-term unresponsive state to the specific immunity, diffusion of which might cause a wider range of immune tolerance [167]. HLA-G directly impaired the cytotoxicity of effector T cells [168], mitigating the anti-tumor activity of γδΤ cells [169] and facilitating the immune evasion of target cells. Additionally, the chemotaxis of T cells was affected by sHLA-G via different mechanisms. sHLA-G reduced CXCR3 expression on CD8+ T cells and γδΤ cells to dampen the response to CXCL10 and CXCL11-mediated chemotaxis. The chemotaxis of CD4+ T cells induced by CCL2, CCL8, CXCL10, and CXCL11 was severely impaired due to the downregulation of CCR2, CXCR3, and CXCR5, whereas the recruitment of follicular helper T cells (TFH) to CXCL13-expressing regions was hindered on account of decreased CXCR5 expression [170]. To note, sHLA-G derived from macrophages or activated monocytes could drive the polarization of T helper 2 (Th2) cells and promote the secretion of IL-10, IL-4, and IL-3, which in turn further upregulated HLA-G expression. Such positive feedback disrupted the natural equilibrium between Th1 and Th2, leading to a severe immunosuppressive state [171].
Roles of HLA-G in tumor immune microenvironment. HLA-G can inhibit the cytotoxicity and chemotaxis of T cells and foster the differentiation of the CD4 + T cells into Treg cells through direct binding, while sHLA-G can drive Th2 cells polarization and potentiate TIM-3 expression to indirectly exert suppressive effects on T cells. Similarly, HLA-G dampens NK cells cytotoxicity, chemotaxis, and migration, while inducing apoptosis and senescence. Moreover, the interaction between HLA-G and ILTs restrains the proliferation of neutrophils, impairs the proliferation, differentiation, chemotaxis, and antibody secretion of B cells, and triggers a shift in macrophage polarization toward M2. MDSC proliferation and accumulation are facilitated by HLA-G. Additionally, HLA-G impairs the activation and antigen-presenting function of DCs and promotes tolerogenic DCs induction. To note, tumor cells can transfer HLA-G to other tumor cells and effector immune cells, leading to the swift dissemination of immunosuppression. NK cells natural killer NK cells, MDSC myeloid-derived suppressor cell, DCs dendritic cells
NK cells are blessed with powerful non-specific killing capacity and are integral to the innate immune system. In analogy to T cells, HLA-G is implicated in the suppression of cytotoxic effects of NK cells [172,173,174] and is capable of reducing the secretion of IFN-γ substantially [175]. HLA-G, especially HLA-G1 and HLA-G3, could boost HLA-E expression [176, 177] and potently suppress NK cells function via HLA-E/NKG2A interaction [178]. Simultaneously, the inhibitory impact of HLA-G was amplified synergistically due to the mitigation of NK cell activation signal MICA/NKG2D [179]. It has been validated that HLA-G induced over-expression of ILT2, ILT3, ILT4, and KIR2DL4 on NK cells independent of antigen stimulation, which might upregulate the threshold of NK cells activation, favoring the immune evasion of target cells [180]. Moreover, the trans-endothelial migration of NK cells was hindered once HLA-G bound with ILT2 [181]. sHLA-G also decreased CXCR3, CCR2, and CX3CR1 expression on peripheral blood NK cells, impeding their chemotactic responses to CXCL10, CXCL11, CCL2, and CX3CL1 and restraining their recruitment to the lesion [182]. On the other hand, sHLA-G fostered FasL expression and secretion, which triggered apoptosis of CD8+ NK cells [162].
In addition, HLA-G suppressed B cells proliferation, differentiation, and antibody secretion after binding to ILT2, and decreased CXCR4 and CXCR5 expression on B cells in the germinal center to restrict the chemotaxis [183, 184]. Additionally, exposure to sHLA-G5 triggered macrophage polarization towards M2 as evidenced by an increase in CD163 expression and a decrease in CD86 expression [185]. HLA-G modulated DCs differentiation via IL-6/STAT3 signaling pathway [186], fostering the induction of tolerogenic DCs while impairing their activation and antigen-presentation functions [187, 188]. In particular, the tolerogenic subset DC-10 could overexpress HLA-G to induce the generation of Treg cells dependent on IL-10 and inhibit cytokine secretion of activated invariant natural killer T (iNKT) cells, contributing to immune response suppression. In this setting, it is plausible that HLA-G could amplify the immune escape effect with the help of the positive feedback loop formed by DCs [151, 189]. Moreover, the expansion and accumulation of MDSCs were also remarkably promoted in response to HLA-G [153, 190,191,192]. Collectively, HLA-G could impede the functions of effector cells while enhance the activities of suppressive and regulatory cells, supporting the remodeling of the immunosuppressive microenvironment. However, there is an ongoing need for studies regarding the clinical significance of the crosstalk between HLA-G and immune cells, which will aid in the development of effective treatment schemes.
HLA-G interaction with immune checkpoint molecules
Immune checkpoints are a group of immunosuppressive molecules expressed on the surface of immune cells, which are dedicated to regulating immune response and essential for the maintenance of autoimmune tolerance and homeostasis. Nevertheless, immune checkpoint molecules are exploited by tumor cells to impede the tumor-killing effects of immune cells with the goal of evading immune attack. As discussed above, growing evidence has demonstrated that HLA-G could potently blunt the cytotoxicity of immune cells including T cells and NK cells, thus its potential as a novel immune checkpoint is gaining increased attention. However, the discrepancies and connections between HLA-G and other known immune checkpoint molecules remain obscure.
-
1.
PD-L1
Programmed death-ligand 1 (PD-L1), is a 40 kDa transmembrane protein encoded by CD274 gene, representing a crucial suppressor of anti-tumor immunity [193]. HLA-G and PD-L1 exhibited consistent high-expression in pancreatic cancer [64], breast cancer [194], papillary thyroid cancer [195], kidney cancer [12, 196, 197], colorectal cancer [102], oral osteosarcomas [198], and intraoral mucoepidermoid carcinoma [199]. In ovarian and gastric cancer, differentiating agents rendered tumor cells more sensitive to chemotherapy drugs due to the loss of stemness and proliferation capacity. However, expression of HLA-G and PD-L1 was upregulated concomitantly, reshaping the suppressive tumor immune microenvironment, which might be a contributor to the poor efficacy of such drugs [200]. Trastuzumab-induced TGF-β and IFN-γ also facilitated HLA-G and PD-L1 expression on HER2-positive breast cancer cells, contributing to trastuzumab resistance [158]. In non-small cell lung cancer (NSCLC), HLA-G and PD-L1 expression were boosted in a dose-dependent manner by several chemotherapeutic agents, among which pemetrexed promoted the glycosylation of HLA-G and PD-L1 consistently as a belt-and-braces approach to the tumor cell immune escape [201]. Despite the sizable evidence implicating a pronounced overlap in the expression patterns of HLA-G and PD-L1 across different tumors, there is heterogeneity in the areas of expression and levels of expression within the same tumor [196].
Intriguingly, HLA-G and PD-L1 have been reported to share other similarities. Firstly, both could induce apoptosis, namely, sHLA-G binding to CD8 triggered apoptosis in T cells and NK cells through Fas/sFasL pathway [162], whilst PD-L1 expressed on lymphatic endothelial cells (LECs) could elicit apoptosis in tumor-specific CD8+ central memory T cells [202]. These results indicated that both HLA-G and PD-L1 could not only directly reduce the population of effector immune cells, but also restrain the lymphocytes functions by engaging with corresponding receptors, working along both lines to shield tumor cells from immune killing. Secondly, both PD-L1 and HLA-G exist in the form of exosomes to impede immune cell functions. Exosomes are nano-vesicles released by normal or neoplastic cells, containing proteins, metabolites, and other bioactive molecules, participating in physiological processes such as tumor angiogenesis, immune evasion, and long-distance intercellular communication [203]. Exosomal PD-L1 inhibited the proliferation of CD4+ and CD8+ T cells, decreased the abundance of T cells in spleen and lymph nodes, and impaired the NK cells cytotoxicity characterized by reduced secretion of IL-2, IFN-γ, and Granzyme B [204]. Exosomal PD-L1 is more effective than soluble PD-L1 in inducing systemic immunosuppression due to its rich circulating routes and robust inhibitory effect, contributing to the poor response rate to anti-PD-L1 therapy and development of drug resistance in most patients [203]. Indeed, PD-L1-targeted therapy in combination with the inhibition of exosome secretion using genetic means or small molecule drugs has shown promising therapeutic efficacy [205,206,207]. In 2003, Be´atrice Riteau et al. reported the existence of secretory exosomal form of HLA-G for the first time [208], whereas the exosomal HLA-G has not been identified in ascitic and pleural exudates from cancer patients until 2013 [44]. To date, the origin of exosomal HLA-G remains inconclusive, but a previous study suggested it might be derived from stem cell-like cancer cells in renal cancer [209]. Differentiation and maturation of peripheral blood mononuclear cell (PBMC)-derived DC cells were restrained by exosomal HLA-G, as well as the T cells activation mediated by DC cells [209]. However, whether exosomal HLA-G has the exact same immunosuppressive functions as membrane-bound HLA-G remains to be appraised. In addition, higher level of exosomal HLA-G was coupled to the presence of stem cell-like circulating tumor cells and the resulting disease progression and poor prognosis, hinting that modalities targeting exosome inhibition might serve as amplifiers of existing neoadjuvant chemotherapy to benefit breast cancer patients [142]. Finally, both HLA-G and PD-L1 proteins could be transferred intercellularly. Trogocytosis was defined as the transfer of membranal molecules from one cell to another, typically through immune synapses [210]. CD8+ T cells acquired functionally active PD-L1 from neighboring mature DC cells or tumor cells via trogocytosis, rendering them into the fight with surrounding PD1+ T cells and resulting in the diffusion of local immunosuppression [211]. The pattern of HLA-G trogocytosis was more diversified and characterized by rapid, transient, and intercellular contact-dependent. Tumor cells were able to deliver HLA-G to effector immune cells. NK cells that received HLA-G from melanoma cells lost their proliferation and tumor-killing capacity and suppressed other NK cells cytotoxicity locally and transiently [212], while the occurrence of CD3+HLA-Gacq+T cells was significantly correlated with poor prognosis in multiple myeloma [213]. Some activated and resting T cells obtained HLA-G from antigen presenting cells (APCs) by trogocytosis and then transformed into Treg cells, acquiring immunosuppressive functions comparable to those of natural Treg cells [214]. Based on the above results, trogocytosis has been regarded as an emergency response to immune attack for HLA-G-expressing cells and tissues. The extensive immunosuppression was orchestrated due to the rapid spread of HLA-G, which was originally restricted in expression, to more kinds of cells and larger areas, enhancing the capacity of the whole tumor to counteract the immune system. In general, HLA-G and PD-L1 shared some similarities, including undergoing multifaceted and multilayered regulation, being in a process of dynamic changes, having similar intercellular transfer pathways, and exerting potent immunosuppressive functions. Hence, previous immunotherapy paradigms targeting PD-L1 may offer valuable insights for developing treatment options based on HLA-G, however, the characteristics of HLA-G still need to be taken into account.
Notably, a regulatory relationship between PD-L1 and HLA-G was also discovered. Activated CD8+T cells pre-treated with sHLA-G exhibited increased level of ILT-2 protein, accompanied by a significant increase in the expression of CTLA-4, PD-1, and TIM3, suggesting a mutual regulation between HLA-G and other immune checkpoints which might be conducive to the immunosuppressive tumor microenvironment (TME) [215].
-
2. Other immune checkpoint molecules
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is one of the first immune checkpoint molecules studied, acting as a transmembrane receptor on T cells that impairs immune response by binding to the corresponding ligands. In BRAFV600E+ papillary thyroid cancer, HLA-G, CTLA-4, and PD-L1 expression were consistently high and significantly inversely correlated with thyroid differentiation score [195], coinciding with the data in renal cell carcinoma [216].
T cell Ig and ITIM domain (TIGHT), another novel immune checkpoint that has gained growing attention, interact with CD155/CD122 ligands to participate in the complex immune regulatory network [217]. Limited bioinformatics data suggested that, contrary to consensus, increased expression of HLA-G and TIGHT was linked to longer disease-free and overall survival in TNBC patients [218]. However, further validation is required to confirm this finding.
There is insufficient data to clarify the relationship between HLA-G and TIM-3, except for one report suggesting that administration of sHLA-G or extracellular vesicles carrying HLA-G could potentiate TIM-3 expression [215]. Nevertheless, the upregulation of HLA-G and HMGB1, one of the identified TIM-3 ligands, was induced by IL-1β, and HLA-G could further increase HLA-G protein level in dependence on TLR4 in glioma [219]. Although the binding site of TIM-3 to HMGB1 and its potential impacts are poorly investigated, it is reasonable to conceive that drugs targeting TIM-3 could modulate HLA-G expression via TLR4-HMGB1 axis.
Collectively, there remains scarce information to date about the relationship between HLA-G and immune checkpoints. In-depth demonstration is fundamental for better targets selection in combination with HLA-G blockade strategies.
Therapeutics targeting HLA-G in cancer
Immunotherapy represented by anti-PD-1/PD-L1 has revolutionized the paradigm of cancer therapy, however, only about 25% of patients with solid tumors benefit effectively and persistently from anti-PD-1/PD-L1 [7]. Elevated level of sHLA-G in plasma and increased expression of ILT-2 have been implicated as possible mechanisms underlying resistance to anti-PD-1 therapies [220, 221]. In this setting, HLA-G-based therapeutics have emerged, aiming to circumvent the issues. HLA-G highly expressed in renal cancer cells specifically impaired the anti-tumor function of CD8+ILT2+PD1-T cells, rather than CD8+ILT2−PBMC or CD8+PD-1+T cells [222], hinting that blockade of HLA-G might be applicable to the patients who failed to respond to anti-PD-1/PD-L1 therapy, as a complement to existing immunotherapy regimens. The absence of PD-L1 and CTLA-4 and the enrichment of HLA-G in adenoid cystic carcinomas of salivary glands suggested that existing agents targeting PD-L1 or CTLA-4 might be ineffective due to the lack of targets [223]. Instead, targeting HLA-G appears to be a promising therapeutic approach (Fig. 4).
Harnessing HLA-G in cancer immunotherapy. Numerous HLA-G-based therapeutics currently in preclinical or clinical stages could potentially be leveraged to target human cancers. Monoclonal antibodies and CAR-NK cells against HLA-G can restrain tumor growth. Anti-HLA-G antibody also serve as a favorable partner for chemotherapy, ICB, and targeted therapies, including CDKi, ERKi, AKTi, and angiogenesis inhibitors. Moreover, small-molecule inhibitors, miRNA mimics, and PROTACs may provide new opportunities for future applications of HLA-G. CAR-NK cells chimeric antigen receptor NK cells, ICB immune checkpoint blockade, CDKi CDK inhibitors, ERKi ERK inhibitors, AKTi AKT inhibitors, PROTACs proteolysis-targeting chimeras
Owing to the heterogeneity and diversity of the cancer, a multitude of clinical trials have focused on combination treatments in order to develop preferable therapeutic regimens with improved efficacy. However, the existing combination strategies, including anti-CTLA-4, chemotherapy, and targeted therapy, have yielded unsatisfactory synergistic effects and severe side effects [7]. Therefore, further studies on the feasibility and clinical application potential of combining anti-PD-1/PD-L1 with HLA-G blockade would be valuable. According to a recent study, aberrantly activated cytokine signaling boosted HLA-G and PD-L1 expression to impair NK cells cytotoxicity, leading to trastuzumab resistance, whereby the immense therapeutic potential of HLA-G-blocking antibody combined with anti-PD-1/PD-L1 antibody in trastuzumab-resistant breast cancer patients was indicated [158].
In addition to the preclinical researches above, clinical trials in small groups are also in progress, summarized in Table 3. A prospective observational cohort study (NCT04300088) explored the impact of HLA-G expression level on the efficacy of tumor immunotherapies (anti-PD-1/PD-L1 therapy and/or anti-CTLA-4 therapy), providing a strong rationale for demonstrating the relationship between HLA-G and tumor resistance to immunotherapy. In another clinical trial for advanced solid tumors (NCT04991740), the effectiveness of a novel bispecific antibody JNJ-78306358 that binds to CD3 and HLA-G has been evaluated, as well as its safety, immunogenicity, and pharmacokinetic properties. BND-22, a monoclonal antibody targeting ILT-2, has been proven to significantly improve the efficacy of anti-PD-1 monotherapy in vitro, and the related phase I/II clinical trials are currently recruiting patients (NCT04717375) [221]. To note, an open-label, dose-escalation/expansion, and multi-center clinical trial (NCT04485013) is enrolling patients with advanced refractory or resistant solid tumors, including head and neck squamous cell carcinoma, colorectal cancer, non-small cell lung cancer, and triple-negative breast cancer, to be given single-agent TTX-080 (anti-HLA-G antibody), or in combination with the PD-1 inhibitor pembrolizumab or the EGFR inhibitor cetuximab. The study aims to determine the safety and tolerability of TTX-080 and illustrate the feasibility and preliminary efficacy of combining TTX-080 with pembrolizumab or cetuximab. This will provide further insight into the future clinical applications of HLA-G inhibition for the benefit of more cancer patients.
Chemotherapeutic agents retard tumor growth chiefly by arresting cell cycle, inhibiting DNA replication, and disturbing cell metabolism [224], but they could also serve as immunomodulators to induce immunogenic death, eliminate immunosuppressive cells, and activate immune effector cells [225, 226]. In NSCLC, pemetrexed, a multi-targeted chemotherapeutic agent interfering with folate metabolic processes, strengthened the expression of HLA-G and PD-L1 by promoting glycosylation. Therefore, simultaneous targeting of PD-L1 and HLA-G could amplify the efficacy of pemetrexed in vitro [201], having implications for designing rational combination treatments for clinical trials in NSCLC. In metastatic colorectal cancer, irinotecan could be captured by HLA-G proteins, resulting in changes in pharmacokinetics and interference with the interaction between HLA-G and its receptor, but it remains ambiguous whether anti-HLA-G therapies could potentiate or attenuate the efficacy of irinotecan [140]. Most patients would suffer chemo-resistance due to the generation of cancer stem cells, while the differentiation strategies attempted to reverse the resistance by converting the rapidly proliferating malignant tumor cells to benign types [227]. However, ovarian cancer cells treated with differentiation agents showed a decrease in stemness and an increase in HLA-G and PD-L1 expression as a defense against anti-tumor immunity, indicating that the efficacy of combining differentiating drugs with chemotherapeutics might be unsatisfactory and the impact of immunosuppressive TME should be further explored [200]. On this basis, blockade of HLA-G may be a superior partner to chemotherapy for long-term effective cancer control and reversal of drug-resistant tumors.
Derangement of normal cell cycle often occurs in cancer, triggering aberrant activation of cell-cycle proteins, making targeting cyclin-dependent kinases (CDKs), or adjusting the cell cycle a beneficial approach to halt tumor growth [228]. CDK-1 is a key regulator of cell cycle progression, which interacts with Cyclin B to activate cells into M-phase and ensure normal mitosis [229]. Lovastatin synchronizes cancer cells in the G1 phase as with CDK4/6 inhibitor [230]. Nocodazole, on the other hand, prevents mitosis and induces apoptosis in tumor cells [231]. It has been shown that nocodazole and CDK1 inhibitors, but not lovastatin, could cause cell cycle blockade accompanied by increased expression of HLA-G and PD-L1, which impaired the killing effect of immune cells against tumor cells [232]. This finding may partially explain why CDK4/6 inhibitors have a better anti-tumor effect compared to other CDKs. It also suggests that blockade of HLA-G may enhance the efficacy of CDK1 inhibitors and nocodazole.
Hyperactive metabolism and disproportionate blood supplies render the tumor microenvironment hypoxic, leading to the accumulation of vascular endothelial growth factor (VEGF), which fosters angiogenesis and concomitantly arouses the upregulation of multiple immune checkpoints, including HLA-G [29, 233]. Hyperproliferation of disorganized vessels hinders immune cell infiltration to drive the development of immunosuppressive TME [234], whereas angiogenesis inhibitors exerts antitumor effects through vascular normalization and TME remodeling [235, 236]. HLA-G/ILT-4 signaling was crucial to tumor angiogenesis, documented with that the interplay between HLA-G and ILT4 boosted the expression of VEGF-C in clear cell renal cell carcinoma and NSCLC, thereby facilitating the tumor growth and lymphatic metastasis [105, 237, 238]. Thus, a combination of HLA-G blockade and angiogenesis inhibitor has been proposed, yet more compelling evidence is required to assess its efficacy.
Beyond the targeted therapies mentioned above, inhibition of AKT or ERK might coordinate with HLA-G-based therapies, as HLA-G/ILT-4 has been shown to promote tumor cell proliferation, migration, and invasion through the activation of AKT/ERK signaling in CRC [105].
Currently, therapies targeting HLA-G centered on developing specific antibodies to block the conjugation of HLA-G and its receptor, featuring the advantages of high affinity and high selectivity, yet having many shortcomings in terms of stability, immunogenicity, and production costs. In this context, orally-bioavailable small-molecule inhibitors with high drug concentration, minor rejection, and low cost are nominated as more desirable antitumor therapies [239, 240]. However, due to the complex spatial interactions between HLA-G and its ligands, competitive binding sites have not yet been well-characterized, leaving the development of small-molecule inhibitors lagging behind that of antibodies. The proteolysis-targeting chimeras (PROTACs) have revolutionized the development of small molecule drugs by harnessing the natural protein degradation system in the body to reduce protein levels [241]. Given that HLA-G could be ubiquitinated, designing suitable PROTACs linking HLA-G and E3 ligase to form a ternary complex that facilitates recognition and degradation of ubiquitinated HLA-G by the proteasome might be a novel tactic to decrease HLA-G expression. In addition to this, ablation of HLA-G using RNAi or CRISPR/Cas9 gene editing may also potentiate the anti-tumor response. In renal cell carcinoma or choriocarcinoma cells, stronger killing capacity of NK cells against tumor cells was evoked after silencing of HLA-G with the CRISPR/Cas9 system [242]. Interference with HLA-G expression using miRNA mimics diminished the viability, migration, and invasion of OSCC cells [119]. However, these two approaches are still preliminary and their application in the clinic remains to be elucidated.
Chimeric antigen receptors (CARs) are artificial receptor molecules created by genetic engineering technology, which could confer specificity to immune effector cells (e.g., T lymphocytes, NK cells) against antigenic epitope of the target, thereby reinforcing the ability of immune cells in recognizing antigenic signals and activation [243]. Nevertheless, the poor clinical efficacy of CAR-T or CAR-NK therapies may be attributed to the presence of a plethora of immunosuppressive molecules in the TME, including HLA-G. It was demonstrated that the anti-HLA-G constructs convert inhibitory signals into activating signals, initiating robust cytotoxicity of engineered CAR-NK cells upon contact with tumor cells. Low-dose chemotherapy enhanced the sensitivity of tumor cells to CAR-NK cells by raising HLA-G expression on the surface of tumor cells [22]. CAR-NK cells were characterized by higher safety, easier accessibility, and fewer side effects compared with CAR-T cells. Considering that HLA-G is limited to immune-privileged tissues and is almost undetectable in normal cells, chemotherapy-induced high expression of HLA-G in tumor cells would cause a further reduction in the damaging effects of HLA-G CAR-NK on normal tissues.
Therapies targeting HLA-G are emerging as a promising area of clinical research, with preliminary success in certain cancers. However, clinical trials with larger cohorts are needed to validate these early results and identify a safe and effective single-agent or combination modality based on HLA-G that can be widely used in clinical practice.
Conclusions
In this review, we propose that HLA-G, as a novel IC, constructs a complex immune regulatory network by impairing or potentiating the function of key immune cells. The expression pattern of HLA-G in cancers is highly heterogeneous, with an overall trend of increasing with disease progression, suggesting that HLA-G plays an important role in the development of malignancy tumors. Hence, a growing body of preclinical work attempts to unveil novel approaches to break the HLA-G-based shield adopted by tumors to restore the anti-tumor response of immune effector cells, advancing future clinical applications. However, new means or strategies for detecting HLA-G are urgently needed given the poor consistency of existing antibodies or kits, which is of vital importance to illustrate the function of HLA-G and its different transcript isoforms. Furthermore, there are still a number of issues to be resolved in the future. By which mechanisms does HLA-G specifically regulate immune cells or tumor cells to exert immunosuppressive functions? What is the role of HLA-G signaling in the pathogenesis of oncology? What is the efficacy and safety of strategies targeting HLA-G as monotherapy or in combination with existing ICBs such as anti-PD-1/PD-L1 antibodies in the treatment of cancer? Is it feasible to develop antibodies targeting both HLA-G and ILT? How can new potential small-molecule agents be discovered to combat the limitations of monoclonal antibodies by targeting the regulatory mechanisms of HLA-G? Can sHLA-G and exosomal HLA-G be used as reliable blood-based biomarkers for early diagnosis or prediction of responsiveness to chemotherapy and ICB therapies? Despite the aforementioned issues have denoted the need for further evidence to pinpoint the intertwined role of HLA-G in cancers, HLA-G holds immense potential to be a promising biomarker for early diagnosis and prognosis assessment, and constitutes an effective targetable strategy for postponing or halting tumor growth.
Availability of data and materials
Not applicable.
Abbreviations
- ICB:
-
Immune checkpoint blockade
- HLA-G:
-
Human leukocyte antigen-G
- MHC:
-
Major histocompatibility complex
- NK cells:
-
Natural killer NK cells
- MDSC:
-
Myeloid-derived suppressor cell
- DNMT:
-
DNA methyltransferase
- HDACi:
-
Histone deacetylase inhibitor
- TSA:
-
Trichostatin A
- CREB:
-
CAMP-responsive element binding protein
- IRF:
-
Interferon-regulatory factor
- ISRE:
-
Interferon-stimulated response element
- HIF-1:
-
Hypoxia-inducible factor 1
- HRE:
-
Hypoxia responsive element
- HSF1:
-
Heat shock factor 1
- HSE:
-
Heat shock element
- LINE:
-
Long interspersed nuclear elements
- PRE:
-
Progesterone response element
- EGF:
-
Epidermal growth factor
- OSCC:
-
Oral squamous cell carcinoma
- CIN:
-
Cervical intraepithelial neoplasia
- GWAS:
-
Genome-wide association
- TFH :
-
Follicular helper T cells
- Th2:
-
T helper 2
- DCs:
-
Dendritic cells
- iNKT:
-
Invariant natural killer T
- PD-L1:
-
Programmed death-ligand 1
- NSCLC:
-
Non-small cell lung cancer
- LECs:
-
Lymphatic endothelial cells
- PBMC:
-
Peripheral blood mononuclear cell
- APCs:
-
Antigen presenting cells
- TME:
-
Tumor microenvironment
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- TIGHT:
-
T cell Ig and ITIM domain
- TIM-3:
-
T cell immunoglobulin domain and mucin domain-3
- CDK:
-
Cyclin-dependent kinase
- VEGF:
-
Vascular endothelial growth factor
- PROTACs:
-
Proteolysis-targeting chimeras
- CARs:
-
Chimeric antigen receptors
References
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–57.
Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020. https://doi.org/10.3390/cancers12030738.
Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39.
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61.
Kim TK, Vandsemb EN, Herbst RS, Chen L. Adaptive immune resistance at the tumour site: mechanisms and therapeutic opportunities. Nat Rev Drug Discov. 2022;21(7):529–40.
Yang H, Yao Z, Zhou X, Zhang W, Zhang X, Zhang F. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213:108377.
Carosella ED, Rouas-Freiss N, Tronik-Le Roux D, Moreau P, LeMaoult J. HLA-G: an immune checkpoint molecule. Adv Immunol. 2015;127:33–144.
Ozato Y, Kojima Y, Kobayashi Y, Hisamatsu Y, Toshima T, Yonemura Y, et al. Spatial and single-cell transcriptomics decipher the cellular environment containing HLA-G+ cancer cells and SPP1+ macrophages in colorectal cancer. Cell Rep. 2023;42(1):111929.
Lin YC, Hua CH, Lu HM, Huang SW, Chen Y, Tsai MH, et al. CAR-T cells targeting HLA-G as potent therapeutic strategy for EGFR-mutated and overexpressed oral cancer. iScience. 2023;26(3):106089.
Tronik-Le Roux D, Renard J, Verine J, Renault V, Tubacher E, LeMaoult J, et al. Novel landscape of HLA-G isoforms expressed in clear cell renal cell carcinoma patients. Mol Oncol. 2017;11(11):1561–78.
Paul P, Cabestre FA, Ibrahim EC, Lefebvre S, Khalil-Daher I, Vazeux G, et al. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol. 2000;61(11):1138–49.
Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci U S A. 1992;89(9):3947–51.
Schiano C, Benincasa G, Infante T, Franzese M, Castaldo R, Fiorito C, et al. Integrated analysis of DNA methylation profile of HLA-G gene and imaging in coronary heart disease: Pilot study. PLoS ONE. 2020;15(8):e0236951.
Tang Y, Liu H, Li H, Peng T, Gu W, Li X. Hypermethylation of the HLA-G promoter is associated with preeclampsia. Mol Hum Reprod. 2015;21(9):736–44.
Friedrich M, Stoehr C, Jasinski-Bergner S, Hartmann A, Wach S, Wullich B, et al. Characterization of the expression and immunological impact of the transcriptional activator CREB in renal cell carcinoma. J Transl Med. 2020;18(1):371.
Dunker K, Schlaf G, Bukur J, Altermann WW, Handke D, Seliger B. Expression and regulation of non-classical HLA-G in renal cell carcinoma. Tissue Antigens. 2008;72(2):137–48.
Moreau P, Mouillot G, Rousseau P, Marcou C, Dausset J, Carosella ED. HLA-G gene repression is reversed by demethylation. Proc Natl Acad Sci USA. 2003;100(3):1191–6.
Yan WH, Lin AF, Chang CC, Ferrone S. Induction of HLA-G expression in a melanoma cell line OCM-1A following the treatment with 5-aza-2’-deoxycytidine. Cell Res. 2005;15(7):523–31.
Wastowski IJ, Simoes RT, Yaghi L, Donadi EA, Pancoto JT, Poras I, et al. Human leukocyte antigen-G is frequently expressed in glioblastoma and may be induced in vitro by combined 5-aza-2’-deoxycytidine and interferon-gamma treatments: results from a multicentric study. Am J Pathol. 2013;182(2):540–52.
Jan CI, Huang SW, Canoll P, Bruce JN, Lin YC, Pan CM, et al. Targeting human leukocyte antigen G with chimeric antigen receptors of natural killer cells convert immunosuppression to ablate solid tumors. J Immunother Cancer. 2021. https://doi.org/10.1136/jitc-2021-003050.
Polakova K, Bandzuchova E, Tirpakova J, Kuba D, Russ G. Modulation of HLA-G expression. Neoplasma. 2007;54(6):455–62.
Solier C, Mallet V, Lenfant F, Bertrand A, Huchenq A, Le Bouteiller P. HLA-G unique promoter region: functional implications. Immunogenetics. 2001;53(8):617–25.
Gobin SJ, van den Elsen PJ. Transcriptional regulation of the MHC class Ib genes HLA-E, HLA-F, and HLA-G. Hum Immunol. 2000;61(11):1102–7.
Gobin SJ, Biesta P, de Steenwinkel JE, Datema G, van den Elsen PJ. HLA-G transactivation by cAMP-response element-binding protein (CREB). An alternative transactivation pathway to the conserved major histocompatibility complex (MHC) class I regulatory routes. J Biol Chem. 2002;277(42):39525–31.
Lefebvre S, Berrih-Aknin S, Adrian F, Moreau P, Poea S, Gourand L, et al. A specific interferon (IFN)-stimulated response element of the distal HLA-G promoter binds IFN-regulatory factor 1 and mediates enhancement of this nonclassical class I gene by IFN-beta. J Biol Chem. 2001;276(9):6133–9.
Ibrahim EC, Morange M, Dausset J, Carosella ED, Paul P. Heat shock and arsenite induce expression of the nonclassical class I histocompatibility HLA-G gene in tumor cell lines. Cell Stress Chaperones. 2000;5(3):207–18.
Garziera M, Scarabel L, Toffoli G. Hypoxic modulation of HLA-G expression through the metabolic sensor HIF-1 in human cancer cells. J Immunol Res. 2017;2017:4587520.
Rousseau P, Masternak K, Krawczyk M, Reith W, Dausset J, Carosella ED, et al. In vivo, RFX5 binds differently to the human leucocyte antigen-E, -F, and -G gene promoters and participates in HLA class I protein expression in a cell type-dependent manner. Immunology. 2004;111(1):53–65.
Ikeno M, Suzuki N, Kamiya M, Takahashi Y, Kudoh J, Okazaki T. LINE1 family member is negative regulator of HLA-G expression. Nucleic Acids Res. 2012;40(21):10742–52.
Flajollet S, Poras I, Carosella ED, Moreau P. RREB-1 is a transcriptional repressor of HLA-G. J Immunol. 2009;183(11):6948–59.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Jasinski-Bergner S, Stoehr C, Bukur J, Massa C, Braun J, Huttelmaier S, et al. Clinical relevance of miR-mediated HLA-G regulation and the associated immune cell infiltration in renal cell carcinoma. Oncoimmunology. 2015;4(6):e1008805.
Wang X, Li B, Wang J, Lei J, Liu C, Ma Y, et al. Evidence that miR-133a causes recurrent spontaneous abortion by reducing HLA-G expression. Reprod Biomed Online. 2012;25(4):415–24.
Castelli EC, Moreau P, Oya e Chiromatzo A, Mendes-Junior CT, Veiga-Castelli LC, Yaghi L, et al. In silico analysis of microRNAS targeting the HLA-G 3’ untranslated region alleles and haplotypes. Hum Immunol. 2009;70(12):1020–5.
Porto IO, Mendes-Junior CT, Felicio LP, Georg RC, Moreau P, Donadi EA, et al. MicroRNAs targeting the immunomodulatory HLA-G gene: a new survey searching for microRNAs with potential to regulate HLA-G. Mol Immunol. 2015;65(2):230–41.
Manaster I, Goldman-Wohl D, Greenfield C, Nachmani D, Tsukerman P, Hamani Y, et al. MiRNA-mediated control of HLA-G expression and function. PLoS ONE. 2012;7(3):e33395.
Veit TD, Chies JA. Tolerance versus immune response—microRNAs as important elements in the regulation of the HLA-G gene expression. Transpl Immunol. 2009;20(4):229–31.
Bertol BC, Massaro JD, Debortoli G, Santos ALP, de Araujo JNG, Giorgenon TMV, et al. BRAF, TERT and HLA-G status in the papillary thyroid carcinoma: a clinicopathological association study. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms241512459.
Li J, Lin TY, Chen L, Liu Y, Dian MJ, Hao WC, et al. miR-19 regulates the expression of interferon-induced genes and MHC class I genes in human cancer cells. Int J Med Sci. 2020;17(7):953–64.
Friedrich M, Vaxevanis CK, Biehl K, Mueller A, Seliger B. Targeting the coding sequence: opposing roles in regulating classical and non-classical MHC class I molecules by miR-16 and miR-744. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2019-000396.
Emirzeoglu L, Olmez O, Mustafayev FNA, Berber U, Yilmaz I, Celik S, et al. Prognostic value of expression levels of miR-148a, miR-152 and HLA-G in colon cancer. Oncol Lett. 2022;24(1):226.
Alegre E, Rebmann V, Lemaoult J, Rodriguez C, Horn PA, Diaz-Lagares A, et al. In vivo identification of an HLA-G complex as ubiquitinated protein circulating in exosomes. Eur J Immunol. 2013;43(7):1933–9.
Diaz-Lagares A, Alegre E, Gonzalez A. Detection of 3-nitrotyrosine-modified human leukocyte antigen-G in biological fluids. Hum Immunol. 2009;70(12):976–80.
Diaz-Lagares A, Alegre E, LeMaoult J, Carosella ED, Gonzalez A. Nitric oxide produces HLA-G nitration and induces metalloprotease-dependent shedding creating a tolerogenic milieu. Immunology. 2009;126(3):436–45.
McMaster M, Zhou Y, Shorter S, Kapasi K, Geraghty D, Lim KH, et al. HLA-G isoforms produced by placental cytotrophoblasts and found in amniotic fluid are due to unusual glycosylation. J Immunol. 1998;160(12):5922–8.
Hakam MS, Miranda-Sayago JM, Hayrabedyan S, Todorova K, Spencer PS, Jabeen A, et al. Preimplantation factor (PIF) promotes HLA-G, -E, -F, -C expression in JEG-3 choriocarcinoma cells and endogenous progesterone activity. Cell Physiol Biochem. 2017;43(6):2277–96.
Yie SM, Li LH, Li GM, Xiao R, Librach CL. Progesterone enhances HLA-G gene expression in JEG-3 choriocarcinoma cells and human cytotrophoblasts in vitro. Hum Reprod. 2006;21(1):46–51.
Yie SM, Xiao R, Librach CL. Progesterone regulates HLA-G gene expression through a novel progesterone response element. Hum Reprod. 2006;21(10):2538–44.
He X, Dong DD, Yie SM, Yang H, Cao M, Ye SR, et al. HLA-G expression in human breast cancer: implications for diagnosis and prognosis, and effect on allocytotoxic lymphocyte response after hormone treatment in vitro. Ann Surg Oncol. 2010;17(5):1459–69.
Moreau P, Flajollet S, Carosella ED. Non-classical transcriptional regulation of HLA-G: an update. J Cell Mol Med. 2009;13(9B):2973–89.
Akhter A, Faridi RM, Das V, Pandey A, Naik S, Agrawal S. In vitro up-regulation of HLA-G using dexamethasone and hydrocortisone in first-trimester trophoblast cells of women experiencing recurrent miscarriage. Tissue Antigens. 2012;80(2):126–35.
Cross JC, Lam S, Yagel S, Werb Z. Defective induction of the transcription factor interferon-stimulated gene factor-3 and interferon alpha insensitivity in human trophoblast cells. Biol Reprod. 1999;60(2):312–21.
Yang Y, Chu W, Geraghty DE, Hunt JS. Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J Immunol. 1996;156(11):4224–31.
Blanco O, Tirado I, Munoz-Fernandez R, Abadia-Molina AC, Garcia-Pacheco JM, Pena J, et al. Human decidual stromal cells express HLA-G: Effects of cytokines and decidualization. Hum Reprod. 2008;23(1):144–52.
Zidi I, Guillard C, Marcou C, Krawice-Radanne I, Sangrouber D, Rouas-Freiss N, et al. Increase in HLA-G1 proteolytic shedding by tumor cells: a regulatory pathway controlled by NF-kappaB inducers. Cell Mol Life Sci. 2006;63(22):2669–81.
Rebmann V, Regel J, Stolke D, Grosse-Wilde H. Secretion of sHLA-G molecules in malignancies. Semin Cancer Biol. 2003;13(5):371–7.
Sebti Y, Le Friec G, Pangault C, Gros F, Drenou B, Guilloux V, et al. Soluble HLA-G molecules are increased in lymphoproliferative disorders. Hum Immunol. 2003;64(11):1093–101.
Gros F, Sebti Y, de Guibert S, Branger B, Bernard M, Fauchet R, et al. Soluble HLA-G molecules increase during acute leukemia, especially in subtypes affecting monocytic and lymphoid lineages. Neoplasia. 2006;8(3):223–30.
Ugurel S, Rebmann V, Ferrone S, Tilgen W, Grosse-Wilde H, Reinhold U. Soluble human leukocyte antigen–G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer. 2001;92(2):369–76.
Persson G, Bork JBS, Isgaard C, Larsen TG, Bordoy AM, Bengtsson MS, et al. Cytokine stimulation of the choriocarcinoma cell line JEG-3 leads to alterations in the HLA-G expression profile. Cell Immunol. 2020;352:104110.
Svendsen SG, Udsen MS, Daouya M, Funck T, Wu CL, Carosella ED, et al. Expression and differential regulation of HLA-G isoforms in the retinal pigment epithelial cell line, ARPE-19. Hum Immunol. 2017;78(5–6):414–20.
Hiraoka N, Ino Y, Hori S, Yamazaki-Itoh R, Naito C, Shimasaki M, et al. Expression of classical human leukocyte antigen class I antigens, HLA-E and HLA-G, is adversely prognostic in pancreatic cancer patients. Cancer Sci. 2020;111(8):3057–70.
Jorgensen N, Sayed A, Jeppesen HB, Persson G, Weisdorf I, Funck T, et al. Characterization of HLA-G regulation and HLA expression in breast cancer and malignant melanoma cell lines upon IFN-gamma stimulation and inhibition of DNA methylation. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21124307.
Frumento G, Franchello S, Palmisano GL, Nicotra MR, Giacomini P, Loke YW, et al. Melanomas and melanoma cell lines do not express HLA-G, and the expression cannot be induced by gammaIFN treatment. Tissue Antigens. 2000;56(1):30–7.
Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine. 2006;24(3):42–51.
Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–99.
Xu HH, Shi WW, Lin A, Yan WH. HLA-G 3’ untranslated region polymorphisms influence the susceptibility for human papillomavirus infection. Tissue Antigens. 2014;84(2):216–22.
da Silva NCH, Sonon P, Medeiros FS, da Silva MC, Dos Santos Gomes FO, Peixoto CA, et al. Contribution of HLA-G and FOXP3 genes and proteins in the severity of cervical intraepithelial neoplasia during HPV infection. Hum Immunol. 2023;84(8):408–17.
Yang YC, Chang TY, Chen TC, Lin WS, Chang SC, Lee YJ. Human leucocyte antigen-G polymorphisms are associated with cervical squamous cell carcinoma risk in Taiwanese women. Eur J Cancer. 2014;50(2):469–74.
Bortolotti D, Gentili V, Rotola A, Di Luca D, Rizzo R. Implication of HLA-G 3’ untranslated region polymorphisms in human papillomavirus infection. Tissue Antigens. 2014;83(2):113–8.
Medeiros FS, Martins AES, Gomes RG, de Oliveira SAV, Welkovic S, Maruza M, et al. Variation sites at the HLA-G 3’ untranslated region confer differential susceptibility to HIV/HPV co-infection and aneuploidy in cervical cell. PLoS ONE. 2018;13(10):e0204679.
Metcalfe S, Roger M, Faucher MC, Coutlee F, Franco EL, Brassard P. The association between human leukocyte antigen (HLA)-G polymorphisms and human papillomavirus (HPV) infection in Inuit women of northern Quebec. Hum Immunol. 2013;74(12):1610–5.
Ferguson R, Ramanakumar AV, Richardson H, Tellier PP, Coutlee F, Franco EL, et al. Human leukocyte antigen (HLA)-E and HLA-G polymorphisms in human papillomavirus infection susceptibility and persistence. Hum Immunol. 2011;72(4):337–41.
Ibrahim EC, Aractingi S, Allory Y, Borrini F, Dupuy A, Duvillard P, et al. Analysis of HLA antigen expression in benign and malignant melanocytic lesions reveals that upregulation of HLA-G expression correlates with malignant transformation, high inflammatory infiltration and HLA-A1 genotype. Int J Cancer. 2004;108(2):243–50.
Xu HH, Zhang X, Zheng HH, Han QY, Lin AF, Yan WH. Association of HLA-G 3’ UTR polymorphism and expression with the progression of cervical lesions in human papillomavirus 18 infections. Infect Agent Cancer. 2018;13:42.
Miranda LN, Reginaldo FP, Souza DM, Soares CP, Silva TG, Rocha KB, et al. Greater expression of the human leukocyte antigen-G (HLA-G) and interleukin-17 (IL-17) in cervical intraepithelial neoplasia: analytical cross-sectional study. Sao Paulo Med J. 2015;133(4):336–42.
Dong DD, Yang H, Li K, Xu G, Song LH, Fan XL, et al. Human leukocyte antigen-G (HLA-G) expression in cervical lesions: association with cancer progression, HPV 16/18 infection, and host immune response. Reprod Sci. 2010;17(8):718–23.
Li XJ, Zhang X, Lin A, Ruan YY, Yan WH. Human leukocyte antigen-G (HLA-G) expression in cervical cancer lesions is associated with disease progression. Hum Immunol. 2012;73(9):946–9.
Aggarwal R, Sharma M, Mangat N, Suri V, Bhatia T, Kumar P, et al. Understanding HLA-G driven journey from HPV infection to cancer cervix: adding missing pieces to the jigsaw puzzle. J Reprod Immunol. 2020;142:103205.
Dhouioui S, Boujelbene N, Ouzari HI, Tizaoui K, Zidi I. Meta-analysis of HLA-G 14bp insertion/deletion polymorphism and soluble HLA-G revealed an association with digestive cancers initiation and prognosis. Heliyon. 2022;8(7):e09986.
Lu Y, Kweon SS, Tanikawa C, Jia WH, Xiang YB, Cai Q, et al. Large-scale genome-wide association study of East Asians identifies loci associated with risk for colorectal cancer. Gastroenterology. 2019;156(5):1455–66.
Dhouioui S, Laaribi AB, Boujelbene N, Jelassi R, Ben Salah H, Bellali H, et al. Association of HLA-G 3’UTR polymorphisms and haplotypes with colorectal cancer susceptibility and prognosis. Hum Immunol. 2022;83(1):39–46.
Tizaoui K, Jalouli M, Ouzari HI, Harrath AH, Rizzo R, Boujelbene N, et al. 3’UTR-HLA-G polymorphisms and circulating sHLA-G are associated with breast cancer: Evidence from a meta-analysis. Immunol Lett. 2022;248:78–89.
Haghi M, Ranjbar M, Karari K, Samadi-Miandoab S, Eftekhari A, Hosseinpour-Feizi MA. Certain haplotypes of the 3’-UTR region of the HLA-G gene are linked to breast cancer. Br J Biomed Sci. 2021;78(2):87–91.
Vaquero-Yuste C, Juarez I, Molina-Alejandre M, Molanes-Lopez EM, Lopez-Nares A, Suarez-Trujillo F, et al. HLA-G 3’UTR polymorphisms are linked to susceptibility and survival in Spanish gastric adenocarcinoma patients. Front Immunol. 2021;12:698438.
Gambella A, Scabini S, Zoppoli G, De Silvestri A, Pigozzi S, Paudice M, et al. HLA-G as a prognostic marker in stage II/III colorectal cancer: not quite there yet. Histochem Cell Biol. 2022;158(6):535–43.
Chen QY, Zhou WJ, Zhang JG, Zhang X, Han QY, Lin A, et al. Prognostic significance of the immune checkpoint HLA-G/ILT-4 in the survival of patients with gastric cancer. Int Immunopharmacol. 2022;109:108798.
Babay W, Ben Yahia H, Boujelbene N, Zidi N, Laaribi AB, Kacem D, et al. Clinicopathologic significance of HLA-G and HLA-E molecules in Tunisian patients with ovarian carcinoma. Hum Immunol. 2018;79(6):463–70.
Singer G, Rebmann V, Chen YC, Liu HT, Ali SZ, Reinsberg J, et al. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res. 2003;9(12):4460–4.
Zhang X, Han QY, Li JB, Ruan YY, Yan WH, Lin A. Lesion HLA-G5/-G6 isoforms expression in patients with ovarian cancer. Hum Immunol. 2016;77(9):780–4.
Andersson E, Poschke I, Villabona L, Carlson JW, Lundqvist A, Kiessling R, et al. Non-classical HLA-class I expression in serous ovarian carcinoma: correlation with the HLA-genotype, tumor infiltrating immune cells and prognosis. Oncoimmunology. 2016;5(1):e1052213.
Guimaraes MC, Soares CP, Donadi EA, Derchain SF, Andrade LA, Silva TG, et al. Low expression of human histocompatibility soluble leukocyte antigen-G (HLA-G5) in invasive cervical cancer with and without metastasis, associated with papilloma virus (HPV). J Histochem Cytochem. 2010;58(5):405–11.
Barrier BF, Kendall BS, Sharpe-Timms KL, Kost ER. Characterization of human leukocyte antigen-G (HLA-G) expression in endometrial adenocarcinoma. Gynecol Oncol. 2006;103(1):25–30.
Ben Yahia H, Boujelbene N, Babay W, Ben Safta I, Dhouioui S, Zemni I, et al. Expression analysis of immune-regulatory molecules HLA-G, HLA-E and IDO in endometrial cancer. Hum Immunol. 2020;81(6):305–13.
Bijen CB, Bantema-Joppe EJ, de Jong RA, Leffers N, Mourits MJ, Eggink HF, et al. The prognostic role of classical and nonclassical MHC class I expression in endometrial cancer. Int J Cancer. 2010;126(6):1417–27.
Menier C, Prevot S, Carosella ED, Rouas-Freiss N. Human leukocyte antigen-G is expressed in advanced-stage ovarian carcinoma of high-grade histology. Hum Immunol. 2009;70(12):1006–9.
Lin A, Zhu CC, Chen HX, Chen BF, Zhang X, Zhang JG, et al. Clinical relevance and functional implications for human leucocyte antigen-g expression in non-small-cell lung cancer. J Cell Mol Med. 2010;14(9):2318–29.
de Magalhaes K, Silva KR, Gomes NA, Sadissou I, Carvalho GT, Buzellin MA, et al. HLA-G 14 bp In/Del and +3142 C/G genotypes are differentially expressed between patients with grade IV gliomas and controls. Int J Neurosci. 2021;131(4):327–35.
Cai MY, Xu YF, Qiu SJ, Ju MJ, Gao Q, Li YW, et al. Human leukocyte antigen-G protein expression is an unfavorable prognostic predictor of hepatocellular carcinoma following curative resection. Clin Cancer Res. 2009;15(14):4686–93.
Chen QY, Chen YX, Han QY, Zhang JG, Zhou WJ, Zhang X, et al. Prognostic significance of immune checkpoints HLA-G/ILT-2/4 and PD-L1 in colorectal cancer. Front Immunol. 2021;12:679090.
de Figueiredo Feitosa NL, Crispim JC, Zanetti BR, Magalhaes PK, Soares CP, Soares EG, et al. HLA-G is differentially expressed in thyroid tissues. Thyroid. 2014;24(3):585–92.
Lin A, Chen HX, Zhu CC, Zhang X, Xu HH, Zhang JG, et al. Aberrant human leucocyte antigen-G expression and its clinical relevance in hepatocellular carcinoma. J Cell Mol Med. 2010;14(8):2162–71.
Cai Z, Wang L, Han Y, Gao W, Wei X, Gong R, et al. Immunoglobulin-like transcript 4 and human leukocyte antigen-G interaction promotes the progression of human colorectal cancer. Int J Oncol. 2019;54(6):1943–54.
Khodabandeh Shahraki P, Zare Y, Azarpira N, Hosseinzadeh M, Farjadian S. Prognostic value of HLA-G in malignant liver and pancreas lesions. Iran J Immunol. 2018;15(1):28–37.
Chen HX, Lin A, Shen CJ, Zhen R, Chen BG, Zhang X, et al. Upregulation of human leukocyte antigen-G expression and its clinical significance in ductal breast cancer. Hum Immunol. 2010;71(9):892–8.
Li JB, Ruan YY, Hu B, Dong SS, Bi TN, Lin A, et al. Importance of the plasma soluble HLA-G levels for prognostic stratification with traditional prognosticators in colorectal cancer. Oncotarget. 2017;8(30):48854–62.
Zheng J, Xu C, Chu D, Zhang X, Li J, Ji G, et al. Human leukocyte antigen G is associated with esophageal squamous cell carcinoma progression and poor prognosis. Immunol Lett. 2014;161(1):13–9.
Bennedsen ALB, Cai L, Hasselager RP, Ozcan AA, Mohamed KB, Eriksen JO, et al. An exploration of immunohistochemistry-based prognostic markers in patients undergoing curative resections for colon cancer. BMC Cancer. 2022;22(1):62.
Ramos CS, Goncalves AS, Marinho LC, Gomes Avelino MA, Saddi VA, Lopes AC, et al. Analysis of HLA-G gene polymorphism and protein expression in invasive breast ductal carcinoma. Hum Immunol. 2014;75(7):667–72.
Ferns DM, Heeren AM, Samuels S, Bleeker MCG, de Gruijl TD, Kenter GG, et al. Classical and non-classical HLA class I aberrations in primary cervical squamous- and adenocarcinomas and paired lymph node metastases. J Immunother Cancer. 2016;4:78.
Jung YW, Kim YT, Kim SW, Kim S, Kim JH, Cho NH, et al. Correlation of human leukocyte antigen-G (HLA-G) expression and disease progression in epithelial ovarian cancer. Reprod Sci. 2009;16(11):1103–11.
Kleinberg L, Florenes VA, Skrede M, Dong HP, Nielsen S, McMaster MT, et al. Expression of HLA-G in malignant mesothelioma and clinically aggressive breast carcinoma. Virchows Arch. 2006;449(1):31–9.
Xu YF, Lu Y, Cheng H, Jiang J, Xu J, Long J, et al. High expression of human leukocyte antigen-G is associated with a poor prognosis in patients with PDAC. Curr Mol Med. 2015;15(4):360–7.
de Kruijf EM, Sajet A, van Nes JG, Natanov R, Putter H, Smit VT, et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol. 2010;185(12):7452–9.
Murdaca G, Calamaro P, Lantieri F, Pigozzi S, Mastracci L, Grillo F, et al. HLA-G expression in gastric carcinoma: clinicopathological correlations and prognostic impact. Virchows Arch. 2018;473(4):425–33.
Imani R, Seyedmajidi M, Ghasemi N, Moslemi D, Shafaee S, Bijani A. HLA-G expression is associated with an unfavorable prognosis of oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2018;19(9):2527–33.
Zhang Y, Jin X, Wang J. miR-148a modulates the viability, migration and invasion of oral squamous cell carcinoma cells by regulating HLA-G expression. Mol Med Rep. 2019;20(1):795–801.
Zhang RL, Zhang X, Dong SS, Hu B, Han QY, Zhang JG, et al. Predictive value of different proportion of lesion HLA-G expression in colorectal cancer. Oncotarget. 2017;8(64):107441–51.
Zhang Y, Zhao J, Qiu L, Zhang P, Li J, Yang D, et al. Co-expression of ILT4/HLA-G in human non-small cell lung cancer correlates with poor prognosis and ILT4-HLA-G interaction activates ERK signaling. Tumour Biol. 2016;37(8):11187–98.
Wang Y, Ye Z, Meng XQ, Zheng SS. Expression of HLA-G in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10(2):158–63.
Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of human leucocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer. 2007;58(2):267–74.
Ye SR, Yang H, Li K, Dong DD, Lin XM, Yie SM. Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol. 2007;20(3):375–83.
Yie SM, Hu Z. Human leukocyte antigen-G (HLA-G) as a marker for diagnosis, prognosis and tumor immune escape in human malignancies. Histol Histopathol. 2011;26(3):409–20.
Lin A, Zhang X, Zhou WJ, Ruan YY, Xu DP, Wang Q, et al. Human leukocyte antigen-G expression is associated with a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Cancer. 2011;129(6):1382–90.
Xu HH, Wang HL, Xing TJ, Wang XQ. A novel prognostic risk model for cervical cancer based on immune checkpoint HLA-G-driven differentially expressed genes. Front Immunol. 2022;13:851622.
Davidson B, Elstrand MB, McMaster MT, Berner A, Kurman RJ, Risberg B, et al. HLA-G expression in effusions is a possible marker of tumor susceptibility to chemotherapy in ovarian carcinoma. Gynecol Oncol. 2005;96(1):42–7.
Rutten MJ, Dijk F, Savci-Heijink CD, Buist MR, Kenter GG, van de Vijver MJ, et al. HLA-G expression is an independent predictor for improved survival in high grade ovarian carcinomas. J Immunol Res. 2014;2014:274584.
Xu X, Yin S, Wang Y, Zhu Q, Zheng G, Lu Y, et al. LILRB1(+) immune cell infiltration identifies immunosuppressive microenvironment and dismal outcomes of patients with ovarian cancer. Int Immunopharmacol. 2023;119:110162.
Scarabel L, Polesel J, De Mattia E, Buonadonna A, D’Andrea MR, Cecchin E, et al. Association of HLA-G 3’UTR polymorphisms with response to first-line FOLFIRI treatment in metastatic colorectal cancer. Pharmaceutics. 2022. https://doi.org/10.3390/pharmaceutics14122737.
Gan J, Di XH, Yan ZY, Gao YF, Xu HH. HLA-G 3’UTR polymorphism diplotypes and soluble HLA-G plasma levels impact cervical cancer susceptibility and prognosis. Front Immunol. 2022;13:1076040.
Ben Yahia H, Babay W, Bortolotti D, Boujelbene N, Laaribi AB, Zidi N, et al. Increased plasmatic soluble HLA-G levels in endometrial cancer. Mol Immunol. 2018;99:82–6.
Babay W, Boujelbene N, Ben Yahia H, Bortolotti D, Zemni I, Ouzari HI, et al. Prognostic significance of high circulating sHLA-G in ovarian carcinoma. HLA. 2021;98(4):357–65.
Tizaoui K, Jalouli M, Boujelbene N, Harrath AH, Ouzari HI, Rizzo R, et al. The relationship of 3’UTR HLA-G14-bp insertion/deletion and +3142 C/G polymorphisms and soluble HLA-G expression with gynecological cancers: An updated meta-analysis. Immun Inflamm Dis. 2022;10(7):e645.
Mejia-Guarnizo LV, Monroy-Camacho PS, Rincon-Rodriguez DE, Rincon-Riveros A, Martinez-Vargas DA, Huertas-Caro CA, et al. Soluble HLA-G (sHLA-G) measurement might be useful as an early diagnostic biomarker and screening test for gastric cancer. Sci Rep. 2023;13(1):13119.
Zheng N, Wang CX, Zhang X, Du LT, Zhang J, Kan SF, et al. Up-regulation of HLA-G expression in cervical premalignant and malignant lesions. Tissue Antigens. 2011;77(3):218–24.
Tan B, Guo J, Wang L, Wang L, Gan X, Chen B. Expression and change of miR-199b-5p, s HLA-G in thyroid carcinoma. Exp Mol Pathol. 2021;120:104643.
Guo ZY, Lv YG, Wang L, Shi SJ, Yang F, Zheng GX, et al. Predictive value of HLA-G and HLA-E in the prognosis of colorectal cancer patients. Cell Immunol. 2015;293(1):10–6.
Scarabel L, Garziera M, Fortuna S, Asaro F, Toffoli G, Geremia S. Soluble HLA-G expression levels and HLA-G/irinotecan association in metastatic colorectal cancer treated with irinotecan-based strategy. Sci Rep. 2020;10(1):8773.
Lazaro-Sanchez AD, Salces-Ortiz P, Velasquez LI, Orozco-Beltran D, Diaz-Fernandez N, Juarez-Marroqui A. HLA-G as a new tumor biomarker: detection of soluble isoforms of HLA-G in the serum and saliva of patients with colorectal cancer. Clin Transl Oncol. 2020;22(7):1166–71.
Konig L, Kasimir-Bauer S, Hoffmann O, Bittner AK, Wagner B, Manvailer LF, et al. The prognostic impact of soluble and vesicular HLA-G and its relationship to circulating tumor cells in neoadjuvant treated breast cancer patients. Hum Immunol. 2016;77(9):791–9.
Roberti MP, Julia EP, Rocca YS, Amat M, Bravo AI, Loza J, et al. Overexpression of CD85j in TNBC patients inhibits Cetuximab-mediated NK-cell ADCC but can be restored with CD85j functional blockade. Eur J Immunol. 2015;45(5):1560–9.
Hoffmann O, Wormland S, Bittner AK, Holzenbein J, Schwich E, Schramm S, et al. Elevated sHLA-G plasma levels post chemotherapy combined with ILT-2 rs10416697C allele status of the sHLA-G-related receptor predict poorest disease outcome in early triple-negative breast cancer patients. Front Immunol. 2023;14:1188030.
Xu HH, Xie YY, Jun G, Yang Z, Han QY. Dynamic changes of soluble HLA-G and cytokine plasma levels in cervical cancer patients: potential role in cancer progression and immunotherapy. J Cancer Res Clin Oncol. 2023;149(8):4195–204.
Schwich E, Rebmann V, Horn PA, Celik AA, Bade-Doding C, Kimmig R, et al. Vesicular-bound HLA-G as a predictive marker for disease progression in epithelial ovarian cancer. Cancers. 2019. https://doi.org/10.3390/cancers11081106.
Wang Q, Song H, Cheng H, Qi J, Nam G, Tan S, et al. Structures of the four Ig-like domain LILRB2 and the four-domain LILRB1 and HLA-G1 complex. Cell Mol Immunol. 2020;17(9):966–75.
Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens. 2004;64(3):215–25.
Zhao J, Zhong S, Niu X, Jiang J, Zhang R, Li Q. The MHC class I-LILRB1 signalling axis as a promising target in cancer therapy. Scand J Immunol. 2019;90(5):e12804.
Kuroki K, Matsubara H, Kanda R, Miyashita N, Shiroishi M, Fukunaga Y, et al. Structural and functional basis for LILRB immune checkpoint receptor recognition of HLA-G isoforms. J Immunol. 2019;203(12):3386–94.
Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116(6):935–44.
Baudhuin J, Migraine J, Faivre V, Loumagne L, Lukaszewicz AC, Payen D, et al. Exocytosis acts as a modulator of the ILT4-mediated inhibition of neutrophil functions. Proc Natl Acad Sci USA. 2013;110(44):17957–62.
Kostlin N, Ostermeir AL, Spring B, Schwarz J, Marme A, Walter CB, et al. HLA-G promotes myeloid-derived suppressor cell accumulation and suppressive activity during human pregnancy through engagement of the receptor ILT4. Eur J Immunol. 2017;47(2):374–84.
Kang X, Kim J, Deng M, John S, Chen H, Wu G, et al. Inhibitory leukocyte immunoglobulin-like receptors: immune checkpoint proteins and tumor sustaining factors. Cell Cycle. 2016;15(1):25–40.
Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, et al. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38(9):637–60.
Faure M, Long EO. KIR2DL4 (CD158d), an NK cell-activating receptor with inhibitory potential. J Immunol. 2002;168(12):6208–14.
Moradi S, Berry R, Pymm P, Hitchen C, Beckham SA, Wilce MC, et al. The structure of the atypical killer cell immunoglobulin-like receptor, KIR2DL4. J Biol Chem. 2015;290(16):10460–71.
Zheng G, Guo Z, Li W, Xi W, Zuo B, Zhang R, et al. Interaction between HLA-G and NK cell receptor KIR2DL4 orchestrates HER2-positive breast cancer resistance to trastuzumab. Signal Transduct Target Ther. 2021;6(1):236.
Fons P, Chabot S, Cartwright JE, Lenfant F, L’Faqihi F, Giustiniani J, et al. Soluble HLA-G1 inhibits angiogenesis through an apoptotic pathway and by direct binding to CD160 receptor expressed by endothelial cells. Blood. 2006;108(8):2608–15.
Fournel S, Aguerre-Girr M, Huc X, Lenfant F, Alam A, Toubert A, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol. 2000;164(12):6100–4.
Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100(15):8856–61.
Contini P, Ghio M, Poggi A, Filaci G, Indiveri F, Ferrone S, et al. Soluble HLA-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol. 2003;33(1):125–34.
Bainbridge DR, Ellis SA, Sargent IL. HLA-G suppresses proliferation of CD4(+) T-lymphocytes. J Reprod Immunol. 2000;48(1):17–26.
Bahri R, Hirsch F, Josse A, Rouas-Freiss N, Bidere N, Vasquez A, et al. Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J Immunol. 2006;176(3):1331–9.
Le Rond S, Azema C, Krawice-Radanne I, Durrbach A, Guettier C, Carosella ED, et al. Evidence to support the role of HLA-G5 in allograft acceptance through induction of immunosuppressive/ regulatory T cells. J Immunol. 2006;176(5):3266–76.
Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–22.
LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED. HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA. 2004;101(18):7064–9.
Le Gal FA, Riteau B, Sedlik C, Khalil-Daher I, Menier C, Dausset J, et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol. 1999;11(8):1351–6.
Lesport E, Baudhuin J, Sousa S, LeMaoult J, Zamborlini A, Rouas-Freiss N, et al. Inhibition of human gamma delta [corrected] T-cell antitumoral activity through HLA-G: implications for immunotherapy of cancer. Cell Mol Life Sci. 2011;68(20):3385–99.
Morandi F, Ferretti E, Bocca P, Prigione I, Raffaghello L, Pistoia V. A novel mechanism of soluble HLA-G mediated immune modulation: downregulation of T cell chemokine receptor expression and impairment of chemotaxis. PLoS ONE. 2010;5(7):e11763.
Carosella ED, Moreau P, Aractingi S, Rouas-Freiss N. HLA-G: a shield against inflammatory aggression. Trends Immunol. 2001;22(10):553–5.
Rouas-Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA. 1997;94(21):11520–5.
Lin A, Xu HH, Xu DP, Zhang X, Wang Q, Yan WH. Multiple steps of HLA-G in ovarian carcinoma metastasis: alter NK cytotoxicity and induce matrix metalloproteinase-15 (MMP-15) expression. Hum Immunol. 2013;74(4):439–46.
Chen BG, Xu DP, Lin A, Yan WH. NK cytolysis is dependent on the proportion of HLA-G expression. Hum Immunol. 2013;74(3):286–9.
Favier B, Lemaoult J, Lesport E, Carosella ED. ILT2/HLA-G interaction impairs NK-cell functions through the inhibition of the late but not the early events of the NK-cell activating synapse. FASEB J. 2010;24(3):689–99.
Teklemariam T, Zhao L, Hantash BM. Full-length HLA-G1 and truncated HLA-G3 differentially increase HLA-E surface localization. Hum Immunol. 2012;73(9):898–905.
Ulbrecht M, Maier S, Hofmeister V, Falk CS, Brooks AG, McMaster MT, et al. Truncated HLA-G isoforms are retained in the endoplasmic reticulum and insufficiently provide HLA-E ligands. Hum Immunol. 2004;65(3):200–8.
Kaiser BK, Pizarro JC, Kerns J, Strong RK. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc Natl Acad Sci USA. 2008;105(18):6696–701.
Menier C, Riteau B, Carosella ED, Rouas-Freiss N. MICA triggering signal for NK cell tumor lysis is counteracted by HLA-G1-mediated inhibitory signal. Int J Cancer. 2002;100(1):63–70.
LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 2005;19(6):662–4.
Dorling A, Monk NJ, Lechler RI. HLA-G inhibits the transendothelial migration of human NK cells. Eur J Immunol. 2000;30(2):586–93.
Morandi F, Ferretti E, Castriconi R, Dondero A, Petretto A, Bottino C, et al. Soluble HLA-G dampens CD94/NKG2A expression and function and differentially modulates chemotaxis and cytokine and chemokine secretion in CD56bright and CD56dim NK cells. Blood. 2011;118(22):5840–50.
Naji A, Menier C, Morandi F, Agaugue S, Maki G, Ferretti E, et al. Binding of HLA-G to ITIM-bearing Ig-like transcript 2 receptor suppresses B cell responses. J Immunol. 2014;192(4):1536–46.
Morandi F, Rouas-Freiss N, Pistoia V. The emerging role of soluble HLA-G in the control of chemotaxis. Cytokine Growth Factor Rev. 2014;25(3):327–35.
Lee CL, Guo Y, So KH, Vijayan M, Guo Y, Wong VH, et al. Soluble human leukocyte antigen G5 polarizes differentiation of macrophages toward a decidual macrophage-like phenotype. Hum Reprod. 2015;30(10):2263–74.
Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc Natl Acad Sci U S A. 2008;105(24):8357–62.
Gros F, Cabillic F, Toutirais O, Maux AL, Sebti Y, Amiot L. Soluble HLA-G molecules impair natural killer/dendritic cell crosstalk via inhibition of dendritic cells. Eur J Immunol. 2008;38(3):742–9.
Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35(4):1133–42.
Wu CL, Caumartin J, Amodio G, Anna F, Loustau M, Gregori S, et al. Inhibition of iNKT cells by the HLA-G-ILT2 checkpoint and poor stimulation by HLA-G-expressing tolerogenic DC. Front Immunol. 2020;11:608614.
Loumagne L, Baudhuin J, Favier B, Montespan F, Carosella ED, Rouas-Freiss N. In vivo evidence that secretion of HLA-G by immunogenic tumor cells allows their evasion from immunosurveillance. Int J Cancer. 2014;135(9):2107–17.
Zhang W, Liang S, Wu J, Horuzsko A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation. 2008;86(8):1125–34.
Agaugue S, Carosella ED, Rouas-Freiss N. Role of HLA-G in tumor escape through expansion of myeloid-derived suppressor cells and cytokinic balance in favor of Th2 versus Th1/Th17. Blood. 2011;117(26):7021–31.
Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48(3):434–52.
Steven A, Seliger B. The role of immune escape and immune cell infiltration in breast cancer. Breast Care. 2018;13(1):16–21.
Na KJ, Choi H. Immune landscape of papillary thyroid cancer and immunotherapeutic implications. Endocr Relat Cancer. 2018;25(5):523–31.
Rouas-Freiss N, LeMaoult J, Verine J, Tronik-Le Roux D, Culine S, Hennequin C, et al. Intratumor heterogeneity of immune checkpoints in primary renal cell cancer: focus on HLA-G/ILT2/ILT4. Oncoimmunology. 2017;6(9):e1342023.
Tronik-Le Roux D, Sautreuil M, Bentriou M, Verine J, Palma MB, Daouya M, et al. Comprehensive landscape of immune-checkpoints uncovered in clear cell renal cell carcinoma reveals new and emerging therapeutic targets. Cancer Immunol Immunother. 2020;69(7):1237–52.
Costa Arantes DA, Goncalves AS, Jham BC, Duarte ECB, de Paula EC, de Paula HM, et al. Evaluation of HLA-G, HLA-E, and PD-L1 proteins in oral osteosarcomas. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(6):e188–96.
Mosconi C, Arantes DAC, Goncalves AS, Alencar RCG, Oliveira JC, Silva TA, et al. Immunohistochemical investigations on the expression of programmed cell death ligand 1, human leukocyte antigens G and E, and granzyme B in intraoral mucoepidermoid carcinoma. Arch Oral Biol. 2017;83:55–62.
Ullah M, Meziani S, Shah S, Kaci R, Pimpie C, Pocard M, et al. Differentiation of cancer cells upregulates HLA-G and PD-L1. Oncol Rep. 2020;43(6):1797–804.
Chen MC, Hung MY, Pan CM, Huang SW, Jan CI, Li YH, et al. Pemetrexed combined with dual immune checkpoint blockade enhances cytotoxic T lymphocytes against lung cancer. Cancer Sci. 2023;114(7):2761–73.
Cousin N, Cap S, Dihr M, Tacconi C, Detmar M, Dieterich LC. Lymphatic PD-L1 expression restricts tumor-specific CD8(+) T-cell responses. Cancer Res. 2021;81(15):4133–44.
Morrissey SM, Yan J. Exosomal PD-L1: roles in tumor progression and immunotherapy. Trends Cancer. 2020;6(7):550–8.
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6.
Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia W, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–4.
Kim DH, Kim H, Choi YJ, Kim SY, Lee JE, Sung KJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med. 2019;51(8):1–13.
Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–27.
Riteau B, Faure F, Menier C, Viel S, Carosella ED, Amigorena S, et al. Exosomes bearing HLA-G are released by melanoma cells. Hum Immunol. 2003;64(11):1064–72.
Grange C, Tapparo M, Tritta S, Deregibus MC, Battaglia A, Gontero P, et al. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer. 2015;15:1009.
Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5(4):261–9.
Gary R, Voelkl S, Palmisano R, Ullrich E, Bosch JJ, Mackensen A. Antigen-specific transfer of functional programmed death ligand 1 from human APCs onto CD8+ T cells via trogocytosis. J Immunol. 2012;188(2):744–52.
Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, et al. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26(5):1423–33.
Brown R, Kabani K, Favaloro J, Yang S, Ho PJ, Gibson J, et al. CD86+ or HLA-G+ can be transferred via trogocytosis from myeloma cells to T cells and are associated with poor prognosis. Blood. 2012;120(10):2055–63.
LeMaoult J, Caumartin J, Daouya M, Favier B, Le Rond S, Gonzalez A, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109(5):2040–8.
Schwich E, Ho GT, LeMaoult J, Bade-Doding C, Carosella ED, Horn PA, et al. Soluble HLA-G and HLA-G bearing extracellular vesicles affect ILT-2 positive and ILT-2 negative CD8 T cells complementary. Front Immunol. 2020;11:2046.
Xu HH, Gan J, Xu DP, Li L, Yan WH. Comprehensive transcriptomic analysis reveals the role of the immune checkpoint HLA-G molecule in cancers. Front Immunol. 2021;12:614773.
Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2020-000957.
Martinez-Canales S, Cifuentes F, De Rodas Lopez, Gregorio M, Serrano-Oviedo L, Galan-Moya EM, Amir E, et al. Transcriptomic immunologic signature associated with favorable clinical outcome in basal-like breast tumors. PLoS ONE. 2017;12(5):e0175128.
Gupta P, Ghosh S, Nagarajan A, Mehta VS, Sen E. beta-defensin-3 negatively regulates TLR4-HMGB1 axis mediated HLA-G expression in IL-1beta treated glioma cells. Cell Signal. 2013;25(3):682–9.
Rebmann* V, Eberhardt* WE, Rizvi N, Antonia SJ, Planchard D, Cappuzzo F, et al. Abstract CT126: soluble HLA-G and -E (sHLA-G/E) as potential biomarkers of clinical outcomes in patients (pts) with advanced, refractory squamous (SQ) NSCLC treated with nivolumab (NIVO): CheckMate. Cancer Res. 2017;77(13):126.
Mandel I, Haves Ziv D, Goldshtein I, Peretz T, Alishekevitz D, Fridman Dror A, et al. BND-22, a first-in-class humanized ILT2-blocking antibody, promotes antitumor immunity and tumor regression. J Immunother Cancer. 2022. https://doi.org/10.1136/jitc-2022-004859.
Dumont C, Jacquier A, Verine J, Noel F, Goujon A, Wu CL, et al. CD8(+)PD-1(-)ILT2(+) T cells are an intratumoral cytotoxic population selectively inhibited by the immune-checkpoint HLA-G. Cancer Immunol Res. 2019;7(10):1619–32.
Mosconi C, de Arruda JAA, de Farias ACR, Oliveira GAQ, de Paula HM, Fonseca FP, et al. Immune microenvironment and evasion mechanisms in adenoid cystic carcinomas of salivary glands. Oral Oncol. 2019;88:95–101.
Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61.
Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–41.
Vinogradov S, Wei X. Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine. 2012;7(4):597–615.
Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39(6):759–78.
Enserink JM, Kolodner RD. An overview of Cdk1-controlled targets and processes. Cell Div. 2010;5:11.
Javanmoghadam-Kamrani S, Keyomarsi K. Synchronization of the cell cycle using lovastatin. Cell Cycle. 2008;7(15):2434–40.
Jackman J, O’Connor PM. Methods for synchronizing cells at specific stages of the cell cycle. Curr Protoc Cell Biol. 2001. https://doi.org/10.1002/0471143030.cb0803s00.
Ullah M, Aoudjeghout W, Pimpie C, Pocard M, Mirshahi M. Mitosis in cancer cell increases immune resistance via high expression of HLA-G and PD-L1. Cancers. 2020. https://doi.org/10.3390/cancers12092661.
Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49.
Bockelmann LC, Schumacher U. Targeting tumor interstitial fluid pressure: will it yield novel successful therapies for solid tumors? Expert Opin Ther Targets. 2019;23(12):1005–14.
Pan C, Liu H, Robins E, Song W, Liu D, Li Z, et al. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol. 2020;13(1):29.
Viallard C, Larrivee B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–26.
Garcia M, Palma MB, Verine J, Miriuka S, Inda AM, Errecalde AL, et al. The immune-checkpoint HLA-G/ILT4 is involved in the regulation of VEGF expression in clear cell renal cell carcinoma. BMC Cancer. 2020;20(1):624.
Su JL, Yang PC, Shih JY, Yang CY, Wei LH, Hsieh CY, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9(3):209–23.
Wang M, Liu Y, Cheng Y, Wei Y, Wei X. Immune checkpoint blockade and its combination therapy with small-molecule inhibitors for cancer treatment. Biochim Biophys Acta Rev Cancer. 2019;1871(2):199–224.
Sasikumar PG, Ramachandra M. Small-molecule immune checkpoint inhibitors targeting PD-1/PD-L1 and other emerging checkpoint pathways. BioDrugs. 2018;32(5):481–97.
Khan S, He Y, Zhang X, Yuan Y, Pu S, Kong Q, et al. PROteolysis TArgeting Chimeras (PROTACs) as emerging anticancer therapeutics. Oncogene. 2020;39(26):4909–24.
Palma MB, Tronik-Le Roux D, Amin G, Castaneda S, Mobbs AM, Scarafia MA, et al. HLA-G gene editing in tumor cell lines as a novel alternative in cancer immunotherapy. Sci Rep. 2021;11(1):22158.
Yilmaz A, Cui H, Caligiuri MA, Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J Hematol Oncol. 2020;13(1):168.
Ishibashi K, Kumai T, Ohkuri T, Kosaka A, Nagato T, Hirata Y, et al. Epigenetic modification augments the immunogenicity of human leukocyte antigen G serving as a tumor antigen for T cell-based immunotherapy. Oncoimmunology. 2016;5(6):e1169356.
Engels CC, Charehbili A, van de Velde CJ, Bastiaannet E, Sajet A, Putter H, et al. The prognostic and predictive value of Tregs and tumor immune subtypes in postmenopausal, hormone receptor-positive breast cancer patients treated with adjuvant endocrine therapy: a Dutch TEAM study analysis. Breast Cancer Res Treat. 2015;149(3):587–96.
Zhu CB, Wang CX, Zhang X, Zhang J, Li W. Serum sHLA-G levels: a useful indicator in distinguishing colorectal cancer from benign colorectal diseases. Int J Cancer. 2011;128(3):617–22.
Zeestraten EC, Reimers MS, Saadatmand S, Goossens-Beumer IJ, Dekker JW, Liefers GJ, et al. Combined analysis of HLA class I, HLA-E and HLA-G predicts prognosis in colon cancer patients. Br J Cancer. 2014;110(2):459–68.
Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of HLA-G is associated with prognosis in esophageal squamous cell carcinoma. Am J Clin Pathol. 2007;128(6):1002–9.
Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of human leukocyte antigen G (HLA-G) correlates with poor prognosis in gastric carcinoma. Ann Surg Oncol. 2007;14(10):2721–9.
Du L, Xiao X, Wang C, Zhang X, Zheng N, Wang L, et al. Human leukocyte antigen-G is closely associated with tumor immune escape in gastric cancer by increasing local regulatory T cells. Cancer Sci. 2011;102(7):1272–80.
Tuncel T, Karagoz B, Haholu A, Ozgun A, Emirzeoglu L, Bilgi O, et al. Immunoregulatory function of HLA-G in gastric cancer. Asian Pac J Cancer Prev. 2013;14(12):7681–4.
Park Y, Park Y, Lim HS, Kim YS, Hong DJ, Kim HS. Soluble human leukocyte antigen-G expression in hepatitis B virus infection and hepatocellular carcinoma. Tissue Antigens. 2012;79(2):97–103.
Yan WH, Liu D, Lu HY, Li YY, Zhang X, Lin A. Significance of tumour cell HLA-G5/-G6 isoform expression in discrimination for adenocarcinoma from squamous cell carcinoma in lung cancer patients. J Cell Mol Med. 2015;19(4):778–85.
Ben Amor A, Beauchemin K, Faucher MC, Hamzaoui A, Hamzaoui K, Roger M. Human leukocyte antigen G polymorphism and expression are associated with an increased risk of non-small-cell lung cancer and advanced disease stage. PLoS ONE. 2016;11(8):e0161210.
Goncalves AS, Wastowski IJ, Capeletti LR, Sacono NT, Cortez AP, Valadares MC, et al. The clinicopathologic significance of the expression of HLA-G in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(3):361–8.
Zhou L, Niu ZY, Liang ZY, Zhou WX, You L, Wang MY, et al. HLA-G impairs host immune response and predicts poor prognosis in pancreatic cancer. Am J Transl Res. 2015;7(10):2036–44.
Nunes LM, Ayres FM, Francescantonio IC, Saddi VA, Avelino MA, Alencar Rde C, et al. Association between the HLA-G molecule and lymph node metastasis in papillary thyroid cancer. Hum Immunol. 2013;74(4):447–51.
Dardano A, Rizzo R, Polini A, Stignani M, Tognini S, Pasqualetti G, et al. Soluble human leukocyte antigen-g and its insertion/deletion polymorphism in papillary thyroid carcinoma: novel potential biomarkers of disease? J Clin Endocrinol Metab. 2012;97(11):4080–6.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2020YFC2008000).
Author information
Authors and Affiliations
Contributions
CTZ and SYW designed the outline. SYW and JXW drafted the manuscript. SYW, YQJ, and ML participated in literature searches and discussion. SYW, JXW, and LZ designed the figures and tables. CTZ and QLG supervised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, S., Wang, J., Xia, Y. et al. Harnessing the potential of HLA-G in cancer therapy: advances, challenges, and prospects. J Transl Med 22, 130 (2024). https://doi.org/10.1186/s12967-024-04938-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-04938-w