Abstract
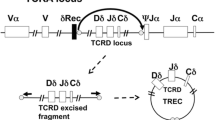
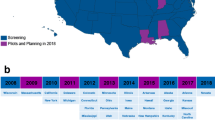
Severe combined immunodeficiency (SCID) is a group of syndromes resulting from genetic defects causing severe deficiency in T cell and B cell function. These conditions are life-threatening and result in susceptibility to serious infections. SCID is often fatal in the first year of life if not detected and properly treated. SCID and related T cell lymphopenias can be detected in newborns by a simple screening test, the T cell receptor excision circle (TREC) assay, using the same dried blood spot samples already collected from newborns to screen for other genetic disorders. The TREC assay facilitates the earliest possible identification of cases of SCID before opportunistic infections, irreversible organ damage, or death, thus allowing for the possibility of curative treatment through hematopoietic stem cell transplant and gene therapy. Infants receiving hematopoietic stem cell transplant in the first few months of life, after being identified through screening, have a high probability of survival (95–100%), along with lower morbidity. The TREC assay has proven to have outstanding specificity and sensitivity to accurately identify almost all infants with SCID (the primary targets) as well as additional infants having other select immunologic abnormalities (secondary targets). The TREC assay is inexpensive and has been effectively integrated into many public health programs. Without timely treatment, SCID is a fatal disease that causes accrual of exorbitant healthcare costs even in just 1 year of life. The cost of care for just one infant with SCID, not diagnosed through newborn screening, could be more than the cost of screening for an entire state or regional population. Continued implementation of TREC screening will undoubtedly enhance early diagnosis, application of treatment, and healthcare cost savings. The Jeffrey Modell Foundation helped initiate newborn screening for SCID in the USA in 2008 and continues its efforts to advocate for SCID screening worldwide. Today, all 50 states and Puerto Rico are screening for SCID and T cell lymphopenia, with 27 million newborns screened to date, and hundreds diagnosed and treated. Additionally, there are at least 20 countries around the world currently conducting screening for SCID at various stages. Newborn screening for SCID and related T cell lymphopenia is cost-effective, and most importantly, it is lifesaving and allows children with SCID the opportunity to live a healthy life.
Similar content being viewed by others
Abbreviations
- US:
-
United States
- PI:
-
Primary immunodeficiency
- SCID:
-
Severe combined immunodeficiency
- HHS:
-
US Department of Health and Human Services
- DOA:
-
US Department of Agriculture
- DOT:
-
US Department of Transportation
- EPA:
-
US Environmental Protection Agency
- OMB:
-
US Office of Management and Budget
- PCR:
-
Polymerase chain reaction
- QALY:
-
Quality of adjusted life year
- NICU:
-
Neonatal intensive care unit
- ADA:
-
Adenosine deaminase
References
Merrill D. No one values your life more than the Federal Government. Bloomberg October 19, 2017. https://www.bloomberg.com/graphics/2017-value-of-life/. Accessed 2 March 2018.
Miller J. The high cost of preventable deaths. June 4, 2018. https://hms.harvard.edu/news/high-cost-preventable-deaths
Mullen A. Immune deficiency disorders: types. Denver: National Jewish Health; 2019. https://www.nationaljewish.org/conditions/immune-deficiency-disorders/types
Amatuni GS, Currier RJ, Church JA, Bishop T, Grimbacher E, Nguyen AA, et al. Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010–2017. Pediatrics. 2019;143(2). https://doi.org/10.1542/peds.2018-2300.
Buckley RH, Schiff SE, Schif RI, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508–16.
Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol Res. 2011;49:25–43.
Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99:872–8.
Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014 August 20;312(7):729–38. https://doi.org/10.1001/jama.2014.9132.
Patel NC, et al. Outcomes of severe combined immunodeficiency patients treated with hematopoietic stem cell transplantation with and without pre-conditioning. J Allergy Clin Immunol. 2009;124(5):1062–9.e1–4.
van der Spek J, Groenwold RH, van der Burg M, van Montfrans JM. TREC based newborn screening for severe combined immunodeficiency disease: a systematic review. J Clin Immunol. 2015;35(4):416–30. https://doi.org/10.1007/s10875-015-0152-6.
Puck JM. Laboratory technology for population-based screening for severe combined immunodeficiency in neonates: the winner is T-cell receptor excision circles. J Allergy Clin Immunol. 2012;129(3):607–16. https://doi.org/10.1016/j.jaci.2012.01.032.
Buckley RH. The multiple causes of human SCID. J Clin Investig. 2004;114:1409–11.
Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–46. https://doi.org/10.1056/NEJMoa1401177.
Haddad, et al. SCID genotype and 6-month posttransplant CD4 count predict survival and immune recovery. Blood. 2018;132(17):1737–49. https://doi.org/10.1182/blood-2018-03-840702.
Baker M. Universal newborn screening for severe combined immunodeficiency (SCID). [PowerPoint]. APHL CDC Newborn Screening Molecular Workshop: Atlanta; 2012.
Korsunskiy I, Blyuss O, Gordukova M, Davydova N, Gordleeva S, Molchanov R, et al. TREC and KREC levels as a predictors of lymphocyte subpopulations measured by flow Cytometry. Front Physiol. 2019;9:1877. https://doi.org/10.3389/fphys.2018.01877.
van der Burg M, Mahlaoui N, Gaspar HB, Pai S-Y. Universal newborn screening for severe combined immunodeficiency (SCID). Front Pediatr. 2019;7:373. https://doi.org/10.3389/fped.2019.00373.
Thakar MS, Hintermeyer MK, Gries MG, Routes JM, Verbsky JW. A practical approach to newborn screening for severe combined immunodeficiency using the T cell receptor excision circle assay. Front Immunol. 2017;8:1470. https://doi.org/10.3389/fimmu.2017.01470.
Bausch-Jurken MT, Verbsky JW, Routes JM. Newborn screening for severe combined immunodeficiency-a history of the TREC assay. Int J Neonatal Screen. 2017;3:14. https://doi.org/10.3390/ijns3020014.
Rechavi E, Lev A, Saraf-Levy T, Etzioni A, Almashanu S, Somech R. Newborn screening for severe combined immunodeficiency in Israel. Int J Neonatal Screen. 2017;3:13. https://doi.org/10.3390/ijns3020013.
Kelly BT, Tam JS, Verbsky JW, Routes JM. Screening for severe combined immunodeficiency in neonates. Clin Epidemiol. 2013;5:363–9. Published 2013 Sep 16. https://doi.org/10.2147/CLEP.S48890.
Adams SP, Rashid S, Premachandra T, Harvey K, Ifederu A, Wilson MC, et al. Screening of neonatal UK dried blood spots using a duplex TREC screening assay. J Clin Immunol. 2014;34:323–30. https://doi.org/10.1007/s10875-014-0007-6.
Dorsey M, Puck J. Screening for severe combined immunodeficiency in the US: current status and approach to management. Int J Neonatal Screen. 2017;3:15.
Modell V, Knaus M, Modell F. An analysis and decision tool to measure cost benefit of newborn screening for severe combined immunodeficiency (SCID) and related T-cell lymphopenia. Immunol Res. 2014;60:145–52. https://doi.org/10.1007/s12026-014-8485-4.
Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol Genet Metab. 2011;104(3):383–9.
Lipstein EA, Vorono S, Browning MF, Green NS, Kemper AR, Knapp AA, et al. Systematic evidence review of newborn screening and treatment of severe combined immunodeficiency. Pediatrics. 2010;125(5):e1226–35. https://doi.org/10.1542/peds.2009-1567.
Hospital Cost and Utilization Project (HCUP), Nationwide Inpatient Database under the auspices of the Agency for Healthcare Research and Quality (AHRQ). ICD-9 CM Principal Diagnosis Code for HSCT.
Centers for Medicare and Medicaid Services, Hospital Accounting Records, April 28, 2010.
Kubiak C, Jyonouchi S, Kuo C, Garcia-Lloret M, Dorsey MJ, Sleasman J, et al. Fiscal implications of newborn screening in the diagnosis of severe combined immunodeficiency. J Allergy Clin Immunol Pract. 2014;2(6):697–702. https://doi.org/10.1016/j.jaip.2014.05.013.
Caggana M, Brower A, Baker M, Comeau AM, Lorey F. National SCID Pilot Study http://preview.tinyurl.com/lsanbqh.
Kuehn BM. State, federal efforts under way to identify children with “bubble boy syndrome”. JAMA. 2010;304(16):1771–3.
Appelbaum B. As US Agencies put more value on a life, businesses fret [newspaper]. New York Times, February 16, 2011. http://tinyurl.com/mlynth7. Accessed 16 Aug 2013.
CBCNews Canada. ‘Bubble boy’ welcomes new Ontario screening test. Published online Aug 20, 2013. http://www.cbc.ca/news/canada/story/2013/08/20/newborn-screening-bubble-boyscid.html. Accessed 21 Aug 2013.
Immunodeficiency Canada. Newborn Screening For SCID. https://immunodeficiency.ca/newborn-screening-for-scid/. Accessed Sept 19, 2019.
Direct communication with Public Health Officials and Jeffrey Modell Centers Network Directors.
Routes JM, Grossman WJ, Verbsky J, Laessig RH, Hoffman GL, Brokopp CD, et al. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302(22):2465–70.
Antoine C, Muller S, Cant A, et al. Long-term survival and transplantation of hemopoietic stem cells for immunodeficiencies; report of the European experience 1986–1999. Lancet. 2003;361(9357):553–60.
Hassan A, Booth C, Brightwell A, Allwood Z, Veys P, Rao K, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120(17):3615–24.
Kwan A, Church JA, Cowan MJ, Agarwal R, Kapoor N, Kohn DB, et al. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia in California: results of the first 2 years. J Allergy Clin Immunol. 2013;132(1):140–50. https://doi.org/10.1016/j.jaci.2013.04.024.
The University of Texas Health Science Center at San Antonio (UTHSCSA). National newborn screening status report. November 21, 2011. http://genes-r-us.uthscsa.edu.
Acknowledgments
The authors profoundly thank the Jeffrey Modell Center Directors for their unwavering and enduring support; and, heartfelt thanks to the patients and their families, who inspire us every day with their courage and fortitude.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest except for the following:
Jordan Orange, MD, PhD, Takeda Consultant, Enzyvant Consultant, received speaking honoraria from Takeda, ADMA Scientific Advisory Board, author and editor in immunology for Up To Date receiving royalties.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quinn, J., Orange, J.S., Modell, V. et al. The case for severe combined immunodeficiency (SCID) and T cell lymphopenia newborn screening: saving lives…one at a time. Immunol Res 68, 48–53 (2020). https://doi.org/10.1007/s12026-020-09117-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-020-09117-9