Abstract
Purpose of Review
This review provides a brief history of newborn screening (NBS) for severe combined immunodeficiency (SCID), discusses the theoretical basis for the T cell receptor excision circle (TREC) assay, highlights the results of recent studies using the TREC, and provides practical advice for the evaluation of infants with an abnormal TREC assay.
Recent Findings
Currently, all but three states perform NBS for SCID in the USA. NBS using the TREC assay is highly sensitive in identifying infants with SCID and may also identify infants with T cell lymphopenia due to other causes such as congenital syndromes, multiple congenital anamolies, and some combined immunodeficiencies.
Summary
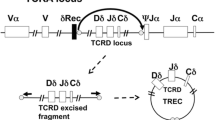
Regardless of the genetic etiology, all forms of SCID are characterized by a severe deficiency of naïve T cells. TRECs are a biomarker of newly formed, naïve T cells that have recently left the thymus. Consequently, the TREC assay identifies infants with SCID and other causes of severe T cell lymphopenia.




Similar content being viewed by others
Abbreviations
- NBS:
-
Newborn screening
- HHS:
-
Health and Human Services
- HSCT:
-
Hematopoietic stem cell transplantation
- SCID:
-
Severe combined immunodeficiency
- TREC:
-
T cell receptor excision circle
- SACHDNC:
-
Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children
- TCR:
-
T cell receptor
- qRT-PCR:
-
Quantitative, real-time polymerase chain reaction
- RUSP:
-
Recommended Uniform Screening Panel
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chinn IK, Shearer WT. Severe combined immunodeficiency disorders. Immunol Allergy Clin N Am. 2015;35:671–94.
•• Kwan A, Abraham RS, Currier R, Brower A, Andruszewski K, Abbott JK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. JAMA. 2014;312:729–38. This study shows the results of NBS of for SCID in over 3 million infants in ten states and the Navajo nation using the TREC assay. A must read for specialists that evaluate infants identified by NBS for SCID.
Wilson JM, Jungner YG. Principles and Practice of Screening for Disease. Public Health Papers. Geneva Switzerland, volume 34. 1968:7–151. http://www.who.int/iris/handle/10665/37650
• Accetta Pedersen DJ, Verbsky J, Routes JM. Screening newborns for primary T-cell immunodeficiencies: consensus and controversy. Expert Rev Clin Immunol. 2011;7:761–8. This article discusses NBS from a public health perspective and reviews some of the controversies involving statewide NBS for SCID and other disorders.
Ding Y, Thompson JD, Kobrynski L, Ojodu J, Zarbalian G, Grosse SD. Cost-effectiveness/cost-benefit analysis of newborn screening for severe combined immune deficiency in Washington State. J Pediatr. 2016;172:127–35.
Chan K, Davis J, Pai SY, Bonilla FA, Puck JM, Apkon M. A Markov model to analyze cost-effectiveness of screening for severe combined immunodeficiency (SCID). Mol Genet Metab. 2011;104:383–9.
Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5.
Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115:391–8.
Baker MW, Grossman WJ, Laessig RH, Hoffman GL, Brokopp CD, Kurtycz DF, et al. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol. 2009;124:8272–9.
•• Routes JM, Grossman WJ, Verbsky J, Laessig RH, Hoffman GL, Brokopp CD, et al. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302:2465–70. Results of the first statewide NBS for SCID, which formed the basis for the addition of SCID to the RUSP by HHS.
Verschuren MC, Wolvers-Tettero IL, Breit TM, Noordzij J, van Wering ER, van Dongen JJ. Preferential rearrangements of the T cell receptor-delta-deleting elements in human T cells. J Immunol. 1997;158:1208–16.
Verbsky JW, Baker MW, Grossman WJ, Hintermeyer M, Dasu T, Bonacci B, et al. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008–2011). J Clin Immunol. 2012;32:82–8.
Mauracher AA, Pagliarulo F, Faes L, Vavassori S, Gungor T, Bachmann LM, et al. Causes of low neonatal T-cell receptor excision circles: a systematic review. J Allergy Clin Immunol Pract. 2017;5:1457–60 e22.
Jyonouchi S, Jongco AM, Puck J, Sullivan KE. Immunodeficiencies associated with abnormal newborn screening for T cell and B cell lymphopenia. J Clin Immunol. 2017;37:363–74.
Morsheimer M, Brown Whitehorn TF, Heimall J, Sullivan KE. The immune deficiency of chromosome 22q11.2 deletion syndrome. Am J Med Genet A. 2017;173:2366–72.
Barry JC, Crowley TB, Jyonouchi S, Heimall J, Zackai EH, Sullivan KE, et al. Identification of 22q11.2 deletion syndrome via newborn screening for severe combined immunodeficiency. J Clin Immunol. 2017;37:476–85.
Albin-Leeds S, Ochoa J, Mehta H, Vogel BH, Caggana M, Bonagura V et. al. Idiopathic T cell lymphopenia identified in New York State Newborn Screening. Clin Immunol. 2017;183:36–40.
Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–46.
Heimall J, Logan BR, Cowan MJ, Notarangelo LD, Griffith LM, Puck JM, et al. Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: a PIDTC natural history study. Blood. 2017;130:2718–27.
• Thakar MS, Hintermeyer MK, Gries MG, Routes JM, Verbsky JWA. Practical Approach to Newborn Screening for Severe Combined Immunodeficiency Using the T Cell Receptor Excision Circle Assay. Front Immunol. 2017;8:1470. This article provides the detailed protocol at Children’s Hospital of Wisconsin for the evaluation and treatment of infants with SCID identified by NBS until the time of HSCT.
Daguindau N, Decot V, Nzietchueng R, Ferrand C, Picard C, Latger-Cannard V, et al. Immune constitution monitoring after PBMC transplantation in complete DiGeorge syndrome: an eight-year follow-up. Clin Immunol. 2008;128:164–71.
Yu H, Zhang VW, Stray-Pedersen A, Hanson IC, Forbes LR, de la Morena MT, et al. Rapid molecular diagnostics of severe primary immunodeficiency determined by using targeted next-generation sequencing. J Allergy Clin Immunol. 2016;138:1142–51 e2.
Acknowledgements
The authors are grateful for the support provided by the Jeffrey Modell Foundation and Children’s Hospital of Wisconsin Foundation in funding the initial statewide, newborn screening for SCID in WI and Emma Cook for her expert editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatric Allergy and Immunology
Rights and permissions
About this article
Cite this article
Routes, J., Verbsky, J. Newborn Screening for Severe Combined Immunodeficiency. Curr Allergy Asthma Rep 18, 34 (2018). https://doi.org/10.1007/s11882-018-0783-9
Published:
DOI: https://doi.org/10.1007/s11882-018-0783-9