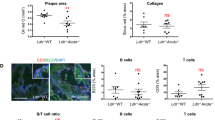
Abstract
The evidence regarding the role of regulatory B cells (Breg) in atherosclerosis are scarce, and there are contradictory data about their atheroprotective properties. Due to the demonstrated protective function of Breg in different inflammatory diseases mainly through interleukin-10 (IL-10) production, the knowledge of their participation in atherosclerosis immunopathology would be very valuable. To further study which B cell subsets participate in IL-10 production and their regulatory role, splenocytes from apolipoprotein-E-deficient mice were evaluated by ex vivo and in vitro cultures. Atherosclerotic mice had increased frequency of IL-10+ B cells, which presented high CD1d, CD19, and IgM, but variable CD5, CD21, and CD23 expression. IL-10+ B cells were not enriched in B cell subsets previously reported as Breg. Increased frequency of IL-10+ B cells with transitional 1-like (T1-like) and follicular (FO) and reduced CD5+ and marginal zone (MZ) phenotypes were observed ex vivo. Increased frequency of IL-10+ B cells with T1-like and MZ, and decreased IL-10+ FO and T2 phenotypes were also observed in vitro. To determine regulatory capacity of B cells in the atherosclerotic model, each subset were co-cultured with CD4+CD25− T cells. CD5+, FO, MZ, and T1-like cells from atherosclerotic mice exhibited regulation in an IL-10-dependent manner. However, only FO cells decreased both frequency of interferon gamma (IFN-γ)+ and tumor necrosis factor alpha (TNF-α)+ and proliferation of T cells. Finally, splenocytes showed increased frequency of IFN-γ+ and TNF-α+ cells only when FO-depleted B cells were evaluated. These results suggest that mainly FO B cells can modulate in some level the inflammatory responses observed in atherosclerosis.





Similar content being viewed by others
References
World Health Organization. World Health Statistics. Geneva: World Health Organization; 2013.
Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–12. doi:10.1038/ni.2001.
McCarthy C, Duffy MM, Mooney D, James WG, Griffin MD, Fitzgerald DJ, et al. IL-10 mediates the immunoregulatory response in conjugated linoleic acid-induced regression of atherosclerosis. FASEB J: Off Publ Fed Am Soc Exp Biol. 2013;27(2):499–510. doi:10.1096/fj.12-215442.
Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90(10):1064–71.
Han X, Kitamoto S, Wang H, Boisvert WA. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J: Off Publ Fed Am Soc Exp Biol. 2010;24(8):2869–80. doi:10.1096/fj.09-148155.
Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9(1–2):10–7.
Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, Groux H, et al. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(8):1474–8. doi:10.1161/01.ATV.0000134378.86443.cd.
Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–50. doi:10.1038/ni833.
Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109(6):745–53. doi:10.1172/JCI7272.
Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203(5):1273–82. doi:10.1084/jem.20052205.
Doran AC, Lipinski MJ, Oldham SN, Garmey JC, Campbell KA, Skaflen MD, et al. B-cell aortic homing and atheroprotection depend on Id3. Circ Res. 2012;110(1):e1–12. doi:10.1161/CIRCRESAHA.111.256438.
Gjurich BN, Taghavie-Moghadam PL, Ley K, Galkina EV. L-selectin deficiency decreases aortic B1a and Breg subsets and promotes atherosclerosis. Thromb Haemost. 2014;112(4):803–11. doi:10.1160/TH13-10-0865.
Major AS, Fazio S, Linton MF. B-lymphocyte deficiency increases atherosclerosis in LDL receptor-null mice. Arterioscler Thromb Vasc Biol. 2002;22(11):1892–8.
Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, et al. B-1b cells secrete Atheroprotective IgM and attenuate atherosclerosis. Circ Res. 2015;117(3):e28–39. doi:10.1161/CIRCRESAHA.117.306044.
Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109(8):830–40. doi:10.1161/CIRCRESAHA.111.248542.
Kyaw T, Tay C, Khan A, Dumouchel V, Cao A, To K, et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. J Immunol. 2010;185(7):4410–9. doi:10.4049/jimmunol.1000033.
Lipinski MJ, Perry HM, Doran AC, Oldham SN, McNamara CA. Comment on “conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis”. J Immunol. 2011;186(1):4; author reply 6. doi:10.4049/jimmunol.1090119.
Rincón-Arévalo H, Yassin-Noreña L, Vásquez G, Castano D. Linfocitos B reguladores en enfermedades humanas y modelos murinos de autoinmunidad. Inmunología. 2013;32(4):129–38.
Rincon-Arevalo H, Sanchez-Parra CC, Castano D, Yassin L, Vasquez G. Regulatory B cells and mechanisms. Int Rev Immunol. 2015; doi:10.3109/08830185.2015.1015719.
Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182(6):3492–502. doi:10.4049/jimmunol.0803052.
Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185(4):2240–52. doi:10.4049/jimmunol.1001307.
Strom AC, Cross AJ, Cole JE, Blair PA, Leib C, Goddard ME, et al. B regulatory cells are increased in hypercholesterolaemic mice and protect from lesion development via IL-10. Thromb Haemost. 2015;114(4):835–47. doi:10.1160/TH14-12-1084.
Sage AP, Nus M, Baker LL, Finigan AJ, Masters LM, Mallat Z. Regulatory B cell-specific interleukin-10 is dispensable for atherosclerosis development in mice. Arterioscler Thromb Vasc Biol. 2015;35(8):1770–3. doi:10.1161/ATVBAHA.115.305568.
Rincon-Arevalo H, Castano D, Villa-Pulgarin J, Rojas M, Vasquez G, Correa LA, et al. Dyslipidemia-associated alterations in B cell subpopulation frequency and phenotype during experimental atherosclerosis. Atherosclerosis. 2016;247:118–26. doi:10.1016/j.atherosclerosis.2015.12.022.
Rincon-Arevalo H, Castano D, Villa-Pulgarin J, Rojas M, Vasquez G, Correa LA, et al. Data in support of dyslipidemia-associated alterations in B cell subpopulations frequency and phenotype during experimental atherosclerosis. Data Brief. 2016;7:958–72. doi:10.1016/j.dib.2016.02.048.
Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol. 2011;677:99–111. doi:10.1007/978-1-60761-869-0_7.
Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491(7423):264–8. doi:10.1038/nature11501.
Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182(12):7459–72. doi:10.4049/jimmunol.0900270.
Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE, et al. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-gamma+CD4+ T cell numbers during colitis development in mice. J Immunol. 2013;191(5):2780–95. doi:10.4049/jimmunol.1300649.
Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–78.
Vadasz Z, Peri R, Eiza N, Slobodin G, Balbir-Gurman A, Toubi E. The expansion of CD25 high IL-10 high FoxP3 high B regulatory cells is in association with SLE disease activity. J Immunol Res. 2015;2015:254245. doi:10.1155/2015/254245.
Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501.
Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189(8):3925–35. doi:10.4049/jimmunol.1103139.
Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–50. doi:10.1016/j.immuni.2008.03.017.
Zhang X. Regulatory functions of innate-like B cells. Cell Mol Immunol. 2013;10(2):113–21. doi:10.1038/cmi.2012.63.
Zhou LJ, Smith HM, Waldschmidt TJ, Schwarting R, Daley J, Tedder TF. Tissue-specific expression of the human CD19 gene in transgenic mice inhibits antigen-independent B-lymphocyte development. Mol Cell Biol. 1994;14(6):3884–94.
Singh AR, Peirce SK, Joshi S, Durden DL. PTEN and PI-3 kinase inhibitors control LPS signaling and the lymphoproliferative response in the CD19+ B cell compartment. Exp Cell Res. 2014;327(1):78–90. doi:10.1016/j.yexcr.2014.05.016.
Moore-Connors JM, Kim HS, Marshall JS, Stadnyk AW, Halperin SA, Wang J. CD43, but not CD43, IL-10-producing CD1dCD5 B cells suppress type 1 immune responses during chlamydia muridarum genital tract infection. Mucosal Immunol. 2014; doi:10.1038/mi.2014.45.
Lee CC, Kung JT. Marginal zone B cell is a major source of Il-10 in listeria monocytogenes susceptibility. J Immunol. 2012;189(7):3319–27. doi:10.4049/jimmunol.1201247.
Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther. 2012;14(1):R32. doi:10.1186/ar3736.
Ledesma-Soto Y, Blanco-Favela F, Fuentes-Panana EM, Tesoro-Cruz E, Hernandez-Gonzalez R, Arriaga-Pizano L, et al. Increased levels of prolactin receptor expression correlate with the early onset of lupus symptoms and increased numbers of transitional-1 B cells after prolactin treatment. BMC Immunol. 2012;13:11. doi:10.1186/1471-2172-13-11.
Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190(1):75–89.
Miyazaki D, Kuo CH, Tominaga T, Inoue Y, Ono SJ. Regulatory function of CpG-activated B cells in late-phase experimental allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2009;50(4):1626–35. doi:10.1167/iovs.08-2701.
Meher AK, Johnston WF, Lu G, Pope NH, Bhamidipati CM, Harmon DB, et al. B2 cells suppress experimental abdominal aortic aneurysms. Am J Pathol. 2014;184(11):3130–41. doi:10.1016/j.ajpath.2014.07.006.
Yoshizaki A, Tedder TF. IL-21 induces regulatory B cell differentiation and immunosuppressive effect through cognate interaction with T cells. Nihon Rinsho Men’eki Gakkai kaishi=Jpn J Clin Immunol. 2015;38(1):57–64. doi:10.2177/jsci.38.57.
Rodgers DT, Pineda MA, McGrath MA, Al-Riyami L, Harnett W, Harnett MM. Protection against collagen-induced arthritis in mice afforded by the parasitic worm product, ES-62, is associated with restoration of the levels of interleukin-10-producing B cells and reduced plasma cell infiltration of the joints. Immunology. 2014;141(3):457–66. doi:10.1111/imm.12208.
Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, Weaver CT, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188(3):1036–48. doi:10.4049/jimmunol.1102500.
Rincon-Arevalo H, Sanchez-Parra CC, Castano D, Yassin L, Vasquez G. Regulatory B cells and mechanisms. Int Rev Immunol. 2016;35(2):156–76. doi:10.3109/08830185.2015.1015719.
Bilate AM, Lafaille JJ. Induced CD4+Foxp3+ regulatory T cells in immune tolerance. Annu Rev Immunol. 2012;30:733–58. doi:10.1146/annurev-immunol-020711-075043.
Acknowledgments
The authors thank Professor Andres Baena (Grupo de Inmunología Celular e Inmunogenética, Universidad de Antioquia, Colombia) for providing us with some of the reagents used in this study. We are also grateful to Julio Jaramillo for their support in animal breeding and care. This work received financial support from Colciencias (project number 111554431390), Sostenibilidad de la Universidad de Antioquia, and the program Jóvenes Investigadores e Innovadores de Colciencias. Professor Lina Yassin was supported by Universidad CES (Facultad de Medicina-Dirección de Gestión del conocimiento).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material

ESM S1
Frequency of B cells secreting IL-10 in atherosclerotic mice. Percentage of IL-10 secreting splenic B cells from WT-HFD and apoE −/−HFD mice after A. ex vivo and B. in vitro cultures. Briefly, isolated B cells were cultured as previously described, incubated for the last 45 min with an anti-IL-10 conjugated to an anti-CD45 antibody to capture secreted IL-10 by each B cell, and finally extracellularly labeled with anti-IL-10. Median and interquartile range of IL-10+ B cells from three to seven mice per group are shown from two independent experiments. Mann-Whitney test. *p < 0.05. (GIF 9 kb)

ESM S2
Frequency of aortic IL-10+ B cells after ex vivo culture. Aortic B cells were cultured ex vivo as explained in the “Materials and methods.” Median and interquartile range from nine to ten mice per group are shown from two independent experiments. Mann-Whitney test. (GIF 3 kb)

ESM S3
Frequency of Foxp3+ cells in IL-10+ B cells region. A. Representative pseudocolor plots of Foxp3 expression in splenic IL-10+ (left) and IL-10− B cells (right) after ex vivo culture. B-C. Frequency of Foxp3+ in IL-10+ and IL-10-splenic B cells after B. ex vivo and C. in vitro cultures. Median and interquartile range from nine mice (WT-HFD and apoE −/−) are shown. D-E. Frequency of Foxp3+ cells in splenic IL-10+ B cells from WT-HFD and apoE −/−HFD mice after D. ex vivo and E. in vitro cultures. Median and interquartile range from four to five mice per group are shown. Mann-Whitney test. ∞ p < 0.0001; Φ p < 0.001; *p < 0.05. (GIF 27 kb)

ESM S4
B cell subsets analysis. Representative pseudocolor plots of CD5+, follicular (FO, CD5−CD21lowCD23+CD24low), transitional 1-like (T1-like, CD5−CD21lowCD23−CD24low), transitional 2 (T2, CD5−CD21hiCD23+CD24hi), and marginal zone (MZ, CD5−CD21hiCD23−CD24hi) B cell subsets from WT-HFD mouse after A ex vivo and B in vitro cultures. (GIF 18 kb)

ESM S5
TNF-α+ and IFN-γ+ T cells analysis. A. Representative pseudocolor plot of gating strategy used in co-cultures and depleted cultures. After exclusion of aggregates (left), cells were selected by negative live/dead expression (center). Only in co-cultures CD4+ were gated (right). B. Representative pseudocolor plot for TNF-α+ and IFN-γ+ cells in CD4+ T cells (from A.) in splenic-enriched T cells from a young WT mice cultured without B cells from apoE −/−HFD mice, as is mentioned in “Materials and methods.” C. Representative analysis of CD4+ T cells proliferation in the co-cultures. Orange non-proliferating CD4+ T cells. Pink proliferating CD4+ T cells. (GIF 68 kb)

ESM S6
Representative pseudocolor plots of enriched B cell subsets from a WT-HFD mouse, according to CD5, CD21, and CD23 markers for the sorting of B cells subsets. A. B cells before sorting. B-F. B cell subsets after sorting. B. CD5+, C. follicular (FO, CD5−CD21lowCD23+CD24low), D. transitional 1-like (T1-like, CD5−CD21lowCD23−CD24low), E. transitional 2 (T2, CD5−CD21hiCD23+CD24hi), and F. marginal zone (MZ, CD5−CD21hiCD23−CD24hi) B cell subsets. (GIF 22 kb)

ESM S7
Proliferation of CD4+CD25− T cells co-cultured with each B cell subsets from atherosclerotic mice. A. Fold change in the proliferation index (total number of divisions per the number of cells that went into division) of CD4+CD25− T cells co-cultured with each B cell subset (CD5+, follicular (FO), marginal zone (MZ), transitional 1-like (T1-like), and transitional 2 (T2)) from apoE −/−HFD mice. B. Fold change in the division index (average number of cell divisions that a cell in the original population has undergone) of T cells co-cultured with B cell subsets from apoE −/−HFD mice. Median and interquartile range from 8 apoE −/−HFD mice for each co-culture are shown from two independent experiments. Two-way ANOVA test with Šídák post-test. Differences between co-cultures and cultures without B cells were indicated as follows: ∞ p < 0.0001; Φ p < 0.001; # p < 0.01; *p < 0.05. Symbol on the bracket indicates difference between co-cultures at a ratio of 1:1 and 2:1. The threshold on the y-axis was stablished based on T cells cultured without B cells. (GIF 27 kb)

ESM S8
Frequencies of B cell subsets after culture. A. Percentage of B cell subsets after A. ex vivo and B. in vitro cultures. Median and interquartile range from 13 to 14 mice per group are shown from two independent experiments. Kruskal-Wallis test with Dunn’s post-test. ∞ p < 0.0001; Φ p < 0.001; # p < 0.01; *p < 0.05. (GIF 48 kb)
Rights and permissions
About this article
Cite this article
Rincón-Arévalo, H., Villa-Pulgarín, J., Tabares, J. et al. Interleukin-10 production and T cell-suppressive capacity in B cell subsets from atherosclerotic apoE −/− mice. Immunol Res 65, 995–1008 (2017). https://doi.org/10.1007/s12026-017-8939-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-017-8939-6