Abstract
Purpose
This study aimed to describe the clinical features, diagnostic and therapeutic course of a patient with MODY13 caused by KCNJ11 (c.101G > A, p.R34H) and how it contributes to the pathogenesis of MODY13, and to explore new therapeutic targets.
Methods
Whole-exome sequencing was used to screen prediagnosed individuals and family members with clinically suspected KCNJ11 mutations. Real-time fluorescence quantitative PCR, western blotting, thallium flux of potassium channels, glucose-stimulated insulin secretion (GSIS), and immunofluorescence assays were used to analyze the regulation of insulin secretion by the KCNJ11 mutant in MIN6 cells. Daily blood glucose levels were continuously monitored for 14 days in the proband using the ambulatory blood glucose meter (SIBIONICS).
Results
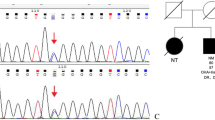
Mutation screening of the entire exon of the gene identified a heterozygous KCNJ11 (c.101G > A, p.R34H) mutation in the proband and his mother. Cell-based GSIS assays after transfection of MIN6 using wild-type and mutant plasmids revealed that this mutation impaired insulin secretory function. Furthermore, we found that this impaired secretory function is associated with reduced functional activity of the mutant KCNJ11 protein and reduced expression of the insulin secretion-associated exocytosis proteins STXBP1 and SNAP25.
Conclusion
For the first time, we revealed the pathogenic mechanism of KCNJ11 (c.101G > A, p.R34H) associated with MODY13. This mutant can cause alterations in KATP channel activity, reduce sensitivity to glucose stimulation, and impair pancreatic β-cell secretory function by downregulating insulin secretion-associated exocytosis proteins. Therefore, oral sulfonylurea drugs can lower blood glucose levels through pro-insulinotropic effects and are more favorable for patients with this mutation.




Similar content being viewed by others
Abbreviations
- ACMG:
-
American College of Medical Genetics
- ALT:
-
albumin transaminase
- AST:
-
aspartate transaminase
- BMI:
-
body mass index
- FBG:
-
fasting blood glucose
- FCP:
-
fasting serum C-peptide
- FINS:
-
fasting serum insulin
- GSIS:
-
glucose stimulated insulin secretion
- HbA1c:
-
hemoglobin A1C
- HDL:
-
high density lipoprotein
- KATP:
-
ATP-sensitive potassium channel
- KCNJ11:
-
potassium inwardly-rectifying channel subfamily J member 11
- LDL:
-
low density lipoprotein
- MODY:
-
maturity-onset diabetes of the young
- MODY13:
-
maturity-onset diabetes mellitus of the young type 13
- SNAP25:
-
synaptosome associated protein 25
- STXBP1:
-
syntaxin binding protein 1
References
A. Bonnefond, J. Philippe, E. Durand et al. Whole-exome sequencing and high throughput genotyping identified KCNJ11 as the thirteenth MODY gene. PLoS ONE 7(6), e37423 (2012)
Y. Kono, M. Horie, M. Takano et al. The properties of the Kir6.1-6.2 tandem channel co-expressed with SUR2A. Pflug. Arch. Eur. J. Physiol. 440(5), 692–698 (2000)
D.L. Cook, C.N. Hales, Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311(5983), 271–273 (1984)
S.L. Shyng, C.G. Nichols, Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science 282(5391), 1138–1141 (1998)
P. Rorsman, F.M. Ashcroft, Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol. Rev. 98(1), 117–214 (2018)
N. Inagaki, T. Gonoi, J.P.T. Clement et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270(5239), 1166–1170 (1995)
S. Haider, J.F. Antcliff, P. Proks, M.S. Sansom, F.M. Ashcroft, Focus on Kir6.2: a key component of the ATP-sensitive potassium channel. J. Mol. Cell. Cardiol. 38(6), 927–936 (2005)
M. Bründl, S. Pellikan, A. Stary-Weinzinger, Simulating PIP(2)-induced gating transitions in Kir6.2 channels. Front. Mol. Biosci. 8, 711975 (2021)
E. De Franco, C. Saint-Martin, K. Brusgaard et al. Update of variants identified in the pancreatic β-cell K(ATP) channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes. Hum. Mutat. 41(5), 884–905 (2020)
K. Shimomura, S.E. Flanagan, B. Zadek et al. Adjacent mutations in the gating loop of Kir6.2 produce neonatal diabetes and hyperinsulinism. EMBO Mol. Med. 1(3), 166–177 (2009)
I. Giurgea, C. Bellanné-Chantelot, M. Ribeiro et al. Molecular mechanisms of neonatal hyperinsulinism. Horm. Res. 66(6), 289–296 (2006)
S.E. Flanagan, A.M. Patch, D.J. Mackay et al. Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 56(7), 1930–1937 (2007)
P. Tammaro, F. Ashcroft, The Kir6.2-F333I mutation differentially modulates KATP channels composed of SUR1 or SUR2 subunits. J. Physiol. 581(Pt 3), 1259–1269 (2007)
B. He, X. Li, Z. Zhou, Continuous spectrum of glucose dysmetabolism due to the KCNJ11 gene mutation—case reports and review of the literature. J. Diab. 13(1), 19–32 (2021)
Y. Chen, X. Hu, J. Cui, M. Zhao, H. Yao, A novel mutation KCNJ11 R136C caused KCNJ11-MODY. Diabetol. Metab. Syndr. 13(1), 91 (2021)
K. Ohkubo, M. Nagashima, Y. Naito et al. Genotypes of the pancreatic beta-cell K-ATP channel and clinical phenotypes of Japanese patients with persistent hyperinsulinaemic hypoglycaemia of infancy. Clin. Endocrinol. 62(4), 458–465 (2005)
H. Ishihara, T. Asano, K. Tsukuda et al. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36(11), 1139–1145 (1993)
F. Zhang, D. Ma, W. Zhao et al. Obesity-induced overexpression of miR-802 impairs insulin transcription and secretion. Nat. Commun. 11(1), 1822 (2020)
K.M. Nkonge, D.K. Nkonge, T.N. Nkonge, The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY). Clin. Diab. Endocrinol. 6(1), 20 (2020)
D.T. Broome, K.M. Pantalone, S.R. Kashyap, L.H. Philipson, Approach to the patient with MODY-monogenic diabetes. J. Clin. Endocrinol. Metab. 106(1), 237–250 (2021)
N.A. ElSayed, G. Aleppo, V.R. Aroda et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care 46(Suppl 1), S19–s40 (2023)
R.F. Knopf, J.W. Conn, S.S. Fajans, J.C. Floyd, E.M. Guntsche, J.A. Rull, Plasma growth hormone response to intravenous administration of amino acids. J. Clin. Endocrinol. Metab. 25, 1140–1144 (1965)
R.B. Tattersall, S.S. Fajans, A difference between the inheritance of classical juvenile-onset and maturity-onset type diabetes of young people. Diabetes 24(1), 44–53 (1975)
R. Aarthy, K. Aston-Mourney, A. Mikocka-Walus et al. Clinical features, complications and treatment of rarer forms of maturity-onset diabetes of the young (MODY)—a review. J. Diabetes Complications 35(1), 107640 (2021)
G. Maltoni, R. Franceschi, V. Di Natale, et al. Next generation sequencing analysis of MODY-X patients: a case report series. J. Pers. Med 12(10), 1613 (2022).
P. Firdous, K. Nissar, S. Ali et al. Genetic testing of maturity-onset diabetes of the young current status and future perspectives. Front. Endocrinol. 9, 253 (2018)
S.S. Fajans, G.I. Bell, MODY: history, genetics, pathophysiology, and clinical decision making. Diabetes Care 34(8), 1878–1884 (2011)
J.W. Kleinberger, K.C. Copeland, R.G. Gandica et al. Monogenic diabetes in overweight and obese youth diagnosed with type 2 diabetes: the TODAY clinical trial. Genet. Med. Off. J. Am. Coll. Med. Genet. 20(6), 583–590 (2018)
R.M. van Dam, B. Hoebee, J.C. Seidell, M.M. Schaap, T.W. de Bruin, E.J. Feskens, Common variants in the ATP-sensitive K+ channel genes KCNJ11 (Kir6.2) and ABCC8 (SUR1) in relation to glucose intolerance: population-based studies and meta-analyses. Diabet. Med. 22(5), 590–598 (2005)
A. Petersmann, M. Nauck, D. Müller-Wieland et al. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 126(7), 406–410 (2018)
O. El Shazly, M.M. Abou El Soud, D.M. El Mikkawy, I. El Ganzoury, A.M. Ibrahim, Endoscopic-assisted achilles tendon reconstruction with free hamstring tendon autograft for chronic rupture of achilles tendon: clinical and isokinetic evaluation. Arthroscopy 30(5), 622–628 (2014)
B.M. Shields, S. Hicks, M.H. Shepherd, K. Colclough, A.T. Hattersley, S. Ellard, Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 53(12), 2504–2508 (2010)
G. Thanabalasingham, A. Pal, M.P. Selwood et al. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care 35(6), 1206–1212 (2012)
H.Y. Aydogan, N. Gul, D.K. Demirci et al. Precision diagnosis of maturity-onset diabetes of the young with next-generation sequencing: findings from the MODY-IST study in adult patients. Omics 26(4), 218–235 (2022)
X. Song, Y. Cao, J. Ye, W. Dai, S. Zhang, S. Ye, A new mutation c.685G > A:p.E229K in the KCNJ11 gene: a case report of maturity-onset diabetes of the young13. Medicine 101(39), e30668 (2022)
R.A. Studer, B.H. Dessailly, C.A. Orengo, Residue mutations and their impact on protein structure and function: detecting beneficial and pathogenic changes. Biochem. J. 449(3), 581–594 (2013)
S.E. Flanagan, A.M. Patch, S. Ellard, Using SIFT and PolyPhen to predict loss-of-function and gain-of-function mutations. Genet. Test. Mol. Biomark. 14(4), 533–537 (2010)
P.C. Ng, S. Henikoff, SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 31(13), 3812–3814 (2003)
L. Brunerova, D. Rahelić, A. Ceriello, J. Broz, Use of oral antidiabetic drugs in the treatment of maturity-onset diabetes of the young: a mini review. Diabetes Metab. Res. Rev. 34(1) (2000). https://doi.org/10.1002/dmrr.2940
T. Urakami, Maturity-onset diabetes of the young (MODY): current perspectives on diagnosis and treatment. Diabetes Metab. Syndr. Obes. Targets Ther. 12, 1047–1056 (2019)
A.K. Ovsyannikova, O.D. Rymar, E.V. Shakhtshneider et al. ABCC8-related maturity-onset diabetes of the young (MODY12): clinical features and treatment perspective. Diabetes Ther. 7(3), 591–600 (2016)
A. Bonnefond, R. Unnikrishnan, A. Doria et al. Monogenic diabetes. Nat. Rev. Dis. Prim. 9(1), 12 (2023)
Acknowledgements
We thank the Endocrine Laboratory of Qilu Hospital, Shandong University for technical support, all authors for their help during clinical data collection and experiments.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 82270845), Double First Class University Plan (Grant No. 26010162914001), and the Major Basic Research Project of Natural Science Foundation of Shandong Province (Grant No. ZR2020ZD15).
Author information
Authors and Affiliations
Contributions
X.L. designed and carried out experiments. J.G., J.Y., Y.Z., and Y.L. assisted with the paper draft. J.C. and Y.S. monitored the progress of experiments and revised the paper. J.S., L.W., and L.X. helped in designing the study. S.Y. and Z.W. helped analyze clinical data. L.C. and X.H. conceived and designed the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures performed in the study involving human participants were performed under the ethical standards of the institutional and/or national research councils and the Declaration of Helsinki. The study was approved by the Ethics Committee of Qilu Hospital of Shandong University (No.: KYLL-202308-033).
Consent to participate and publication
All subjects included in this study voluntarily signed an informed consent form, which was reviewed by the Ethics Committee of Qilu Hospital of Shandong University. The authors affirm that human research participants provided informed consent for publication of the images in Fig. 1.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, X., Gao, J., Yang, J. et al. Clinical and functional characterization of a novel KCNJ11 (c.101G > A, p.R34H) mutation associated with maturity-onset diabetes mellitus of the young type 13. Endocrine (2024). https://doi.org/10.1007/s12020-024-03873-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03873-6