Abstract
Introduction
To evaluate the relationship between the GRI -component of hypoglycemia (CHypo) and hyperglycemia (CHyper)- with diabetes quality of life (DQoL), diabetes-related stress (DDS), perception of hypoglycemia (Clarke Test), visual analogic scale (VAS) and diabetes-knowledge (DKQ2) in T1D.
Methods
Cross-sectional study in 92 patients with T1D under intensive insulin treatment (21.7% CSII) and flash glucose monitoring (isCGM). Clinical, metabolic and glycometric parameters and quality of life/satisfaction questionnaires were analyzed.
Results
92 patients (54.3% male, BMI 25.4 ± 4.5 kg/m2, HbA1c 7.5 ± 1.0%, TIR 53.9 ± 15.9%) with mean age 36.1 ± 12.6years and 17.8 ± 11.3 T1D duration. The mean GRI was 60.6 ± 22.2 with a CHypo and CHyper of 5.9 ± 4.8 and 27.3 ± 14.4, respectively. 19.1% presented a pathological Clarke’s test. Patients with TIR > 70% and GRI < 40 showed better VAS (8.8 ± 1.3 vs 9.3 ± 0.9, p < 0.05) and DDS (46.4 ± 22.1 vs 36.7 ± 16.6, p < 0.05) scores, showing no differences between groups. CHyper > 15 and Chypo > 3.4 were related to worse levels of DQoL (91.1 ± 23.9 vs 76.6 ± 18.6 and 94.6 ± 24.8 vs 79.8 ± 20.1, p < 0.01), DDS(49.8 ± 22.4 vs 35.7 ± 16.5 and 49.8 ± 22.4 vs 35.7 ± 16.5, p < 0.01),and DKQ2 (24.4 ± 4.3 vs 26.8 ± 5.2 and 24.1 ± 4.8 vs 26.0 ± 4.6, p < 0.05), respectively. Worse metabolic control defined by GRI correlated with worse scores in VAS (r = −0.209, p < 0.05), DQoL (r = 0.205, p < 0.05), and DDS (r = 0.205, p < 0.05). No difference was observed in knowledge´s scale. CHyper correlated with worse scores in VAS (r = −0.231, p < 0.05), DQoL (r = 0.422, p < 0.01), and DDS (r = 0.341, p < 0.01) and lower degree of knowledge DKQ2 (r = −0.231, p < 0.05). When analyzing DQoL as a dependent variable in a multiple lineal regression, only age (β = 0.747; p < 0.001) and CHyper (β = 0.717; p < 0.001) maintained statistical significance.
Conclusions
Higher GRI was related to worse quality of life, diabetes-related stress and satisfaction with treatment, analogous to the TIR results.CHyper an Chypo were related to a greater decline in quality of life, diabetes-related stress, and lower satisfaction with treatment.However, in a multiple linear regression, only CHyper maintained statistical significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Data provided by continuous glucose monitoring (CGM) systems have become the preferred form of blood glucose monitoring to achieve adequate control in patients with type 1 diabetes (T1D) [1,2,3]. It is well known that the use of these devices improves glycosylated hemoglobin A1c (HbA1c) and Time in Range (TIR) levels, reducing the number of episodes of acute hypoglycemia and chronic hyperglycemia and glycemic variability, including acute decompensation and hospital admissions [4,5,6,7]. It has also been related to a decrease in the risk of long-term complications and improvement in quality of life of T1D [4, 8,9,10].
The quality of life of patients with T1D involves different factors of the psychosocial sphere such as stress related to the disease, the grade of knowledge or satisfaction with the treatment, among others [11]. The variability of the scales used to evaluate them, together with the fact that not all of them are examined in the different studies, makes it difficult to compare them [9,10,11,12,13]. However, this is an important point to bear in mind, since improvements in the quality of life of T1D are associated with improvements in parameters such as the TIR, and vice versa [10].
Recently, the appearance of the Glycemic Risk Index (GRI) [14] as a new parameter to measure the quality of glycemic control in patients with diabetes has demonstrated its usefulness in adult and pediatric patients in clinical practice [15, 16]. In addition, GRI has proved its simplicity, ease of calculation and interpretation and its good correlation with the other parameters of the CGM, especially with TIR [17, 18]. The GRI consists of two components, one for hypoglycemia (CHypo) and one for hyperglycemia (CHyper). Both components are calculated from the respective times below and above range weighted according to their clinical relevance. In addition, the GRI can be categorized and graphically represented by percentiles (Pc) in five zones, from the best (Pc: 0–20) to the worst (Pc: 80–100) glycemic control.
However, despite the advantages of integrating this new glycemic metric into the Ambulatory Glucose Profile (AGP) report, its recent appearance means that its relationship with the psychosocial sphere of adult patients with T1D has not yet been elucidated.
The aim of the present study was to evaluate the relationship between the GRI and its CHypo and CHyper, with T1D quality of life, diabetes-related stress, perception of hypoglycemia, satisfaction with treatment, and degree of knowledge in a cohort of adult patients with T1D.
Material and methods
Participants
Cross-sectional study in 92 adult patients with T1D with stable control (more than 1 year of diabetes diagnosis and more than 3 months without changes in insulin treatment) on intensive insulin treatment (multiple doses of insulin (MDI) or continuous subcutaneous insulin infusion (CSII)) and flash glucose monitoring (isCGM) (Free Style Libre 2, Abbott Diabetes Care, Witney, UK), under follow-up in a tertiary Hospital.
Procedures
Clinical and glycometric parameters were collected from the isCGM system data download platform of all patients who attended a control visit between June and December 2021, including in the report the last 14 days prior to the visit [19]. Patients with insufficient use of the system (<70%) and those with recent changes in treatment regimen (insulin type or initiation of CSII) or with less than 3 months of isCGM use were excluded. HbA1c was also measured between 7 and 10 days before the patient’s visit by turbidimetric inhibition immunoassay standardized to the National Glycohemoglobin Standardization Program (Roche Diagnostics, Geneva, Switzerland). Glycometric data were defined as mean glucose (mg/dL), glucose management indicator (GMI) (%), coefficient of variation (CV) (%), and percentages of TIR, time above range (TAR) and time below range (TBR) (%). The latter were divided into Very low, when blood glucose was less than 54 mg/dL (<3.0 mmol/L) TBR < 54; Low, between 54 and 70 mg/dL (3.0–3.9 mmol/L) TBR 54–70; in Range, between 71 and 180 mg/dL (4.0–10.0 mmol/L); High, between 181 and 250 mg/dL (10.1–13.9 mmol/L) TAR 180–250; and Very high, above 250 mg/dL (>13.9 mmol/L) TAR > 250. From the data of the different times obtained from the isCGM, the Hypoglycemia and Hyperglycemia components of the GRI were calculated, whose formula is developed as follows: CHypo = (TBR < 54) + (0.8 × TBR 54–70). CHyper = (TAR > 250) + (0.5 × TAR 180–250). GRI = (3.0 × CHypo) + (1.6 × CHyper) [14]. The education level of the patients in the study (divided into primary, secondary and higher education levels) was collected.
The following validated questionnaires were evaluated at the time of the visit, (1) Diabetes mellitus-specific quality of life questionnaire 20 in Spanish version (DQoL) [11]. The DQoL assesses 4 spheres (a) satisfaction (b) impact (c) social/vocational concern and (d) diabetes-related concern. A lower score indicates better quality of life: range 43–215. (2) The Spanish version of the diabetes-related distress scale (DDS) [20]. The DDS assess the level of diabetes-related patient stress in four subscales: emotional burden, physician-related distress, treatment-related distress, and interpersonal distress. The score range is 17 to 102, with the higher score being related to the greater degree of stress. (3) Clarke questionnaire for the perception of hypoglycemia, Spanish version [21] (1–2R normal perception; 3R indeterminate perception; >3R hypoglycemia unnoticed). The diabetes knowledge questionnaire was evaluated in its Spanish version (DKQ2) [22], which estimates the degree of knowledge, the maximum degree being a score of 26. Finally, a global assessment of quality of life was made using the Visual Analog Scale (VAS) from 0 to 10 points, with the maximum quality of life being 10 points.
Statistical analysis
Quantitative variables were expressed as mean and (standard deviation) if normally distributed or as median and [interquartile range] when the distribution was not normal. Qualitative variables were expressed in terms of percentages. Comparison between qualitative variables was performed by the Chi-square test, using Fisher’s exact test where necessary. The normal distribution of quantitative variables was examined using the one-sample Kolmogorov–Smirnov test. The quantitative variables with normal distribution were analyzed using a bilateral Student’s t-test, and non-parametric variables were evaluated by using the Mann–Whitney U test. The association of quantitative variables was calculated using Pearson’s linear correlation coefficient. A stratified analysis of the main variables was performed by level of GRI > 40 (poor glycemic control) or ≤40 (group with better glycemic control), CHypo greater or less than 3.4, CHyper greater or less than 15 and TIR greater or less than 70%, as previously described [10]. Finally, a multiple lineal regression model was used that incorporated as independent variables the effect of CHypo, CHyper, type of treatment, CV, age, sex, years of duration of diabetes and education level on DQoL as dependent variable. For all calculations, a p-probability of less than 0.05 was considered significant. SPSS 23.0 (SPSS Inc., Chicago, IL, USA) and Rstudio version RStudio Team (2022 PBC, Boston, MA) were used for data analysis.
All patients signed an informed consent for their inclusion before participating in the study. The protocol was approved by the Clinical Research Ethics Committee of our Institution, and the study was conducted in accordance with the Declaration of Helsinki.
Results
A total of 92 patients with T1D (54.3% male) were evaluated, with a mean age of 36.1 (12.6) years, 17.8 (11.3) years of T1D evolution and a mean BMI of 25.4 (4.5) kg/m2. The mean HbA1c was 7.5 (1.0)%. 21.7% were under CSII. The mean number of daily scans was 9.8 (5.4) with a mean percentage of device use of 91.2 (10.6)%. The glycometric measures obtained were: mean blood glucose 171.5 (35.8) mg/dl, mean SD 67.4 (20.8) mg/dl, CV 40.4 (7.3)%; TIR 53.9 (15.9)%, TBR < 4 2.4 [0.0–13.0]%; TBR 54–70 4.3 (2.8)%; TAR 180–250 24.1 (8.0)%; TAR > 250 15.2 (12.7)%. Education level of patients of the study was: Primary education: 14.1%, Secondary education: 56.5% and Higher education: 29.3% (Table 1). Differences between CSII and MDI are shown in Supplementary Files.
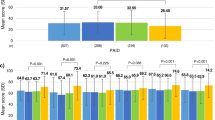
The mean GRI was 60.6 (22.2) with a Hypo and Hyperglycemia Component of 5.9 (4.8) and 27.3 (14.4), respectively. As for the distribution of the GRI by zones, 5.4% occupied zone A (Pc: 0–20), 10.9% zone B (Pc: 21–40), 34.8% zone C (Pc: 41–60), 30.4% zone D (Pc: 61–80) and the remaining 18.5% zone E (Pc: 81–100). The mean scores on the questionnaires were: DQoL 85.8 (23.1), DDS 44.8 (21.5), VAS 8.9 (1.2) and DKQ2 25.2 (4.8). Clarke’s test showed hypoglycemia unawareness in 19.1% of the patients (Table 1 and Fig. 1).
Those patients with better glycemic control define with a TIR > 70% or GRI < 40 showed better scores in the scales: VAS (8.8 ± 1.3 vs 9.3 ± 0.9; p < 0.05) and DDS (46.4 ± 22.1 vs 36.7 ± 16.6; p < 0.05). No differences between TIR and GRI scale results was found. On the other hand, the group with CHyper > 15 (worse control) was related to worse results in the questionnaires: DQoL (91.1 ± 23.9 vs 76.6 ± 18.6; p < 0.01), DDS (49.8 ± 22.4 vs 35.7 ± 16.5; p < 0.01), and DKQ2 (24.4 ± 4.3 vs 26.8 ± 5.2; p < 0.05). Similarly, CHypo > 3.4 was associated to worse results in DQoL (94.6 ± 24.8 vs 79.8 ± 20.1; p < 0.01), DDS (49.8 ± 23.1 vs 41.4 ± 19.9; p < 0.01), and DKQ2 (24.1 ± 4.8 vs 26.0 ± 4.6; p < 0.05) (Table 2).
Worse metabolic control defined by GRI correlated with worse scores in VAS (r = −0.209; p < 0.05), DQoL (r = 0.205; p < 0.05), and DDS (r = 0.205, p < 0.05); parallel to the group with higher TIR: VAS (r = 0.262; p < 0.05), DQoL (r = −0.344; p < 0.01), and DDS (r = −0.313; p < 0.01). No correlation was observed in DKQ2. CHyper was correlated with worse scores in: VAS (r = −0.231; p < 0.05), DQoL (r = 0.422; p < 0.01) and DDS (r = 0.341; p < 0.01), as well as with a lower degree of knowledge DKQ2 (r = −0.231; p < 0.05). CHypo was weakly correlated with DQoL (r = −0.294, p < 0.05) (Fig. 2).
Correlation between glycometric parameters and psychosocial features. GRI glycemia risk index, CHypo component of hypoglycemia, CHyper component of hyperglycemia, TIR time in range, DQoL diabetes quality of life, DDS diabetes distress scale, DKQ2 diabetes knowledge questionnaire 2, VAS visual analogic scale, NS not significant, R Pearson’s correlation coefficient, *P < 0.05. **P < 0.01
No difference was shown in GRI or in the rest of the glycometrics with respect to the positive or negative Clarke test (Supplementary files). There were also no significant differences between questionnaires score in the percentile group (A-E) by GRI. However, there was a tendency towards greater satisfaction with treatment VAS (9.5 ± 0.9 group A vs 8.6 ± 0.8 group E; p = 0.449) and better quality of life DQoL (80.8 ± 26.3 group A vs 85.8 ± 23.2 group E; p = 0.302) in those with better GRI. The diabetes related-stress was higher in the most extreme percentiles DDS (44.2 ± 20.8 group A, 33.0 ± 13.7 group B, 45.6 ± 23.9 group C, 46.1 ± 19.3 group D, 48.5 ± 24.1 group E; p = 0.461), with no differences in DQK2 among the different groups (Table 3).
No differences were observed between the scores on the different questionnaires and educational level, with the exception of the degree of knowledge (DKQ2), where it was found that a higher education level was associated with a higher score on the questionnaire (primary 22; secondary 26.2, and higher education 26.2) (p < 0.05).
Finally, when analyzing DQoL as a dependent variable in a multiple lineal regression model that included CHypo, CHyper, type of treatment, CV, age, sex, years of duration of diabetes and education level as independent variables, the only variables that maintained statistical significance were age (β = 0.747; p < 0.001) and CHyper (β = 0.717; p < 0.001) (Table 4).
Discussion
The GRI as a new parameter of glycemic control has aroused great interest among professionals dedicated to diabetes care [14]. Despite its recent appearance, it has been shown to correlate with glycometric variables such as TIR, TBR, TAR or Coefficient of variation (CV) [17, 18]. In fact, recent studies have related TIR to long-term microvascular complications (retinopathy, nephropathy) [23, 24]. However, its relationship with different aspects of the psychosocial sphere has not been yet studied in adults.
Different authors have already demonstrated the relationship between the TIR and the quality of life of T1D [10, 12, 25]. However, this is to our knowledge the first article that relates the GRI and its two components (hypo and hyperglycemia) to different parameters of the psychosocial sphere in adults with T1D. Our results show how GRI is related to diabetes-related stress (DDS), quality of life (DQoL) and overall treatment satisfaction (VAS) in adult patients with T1D. However, and despite the weighting of the CHypo and CHyper according to their clinical significance [14], this relationship is similar to that obtained by TIR [17, 18]. In fact, the similar results obtained in those patients with better glycemic control (TIR > 70% and GRI < 40) demonstrate the lack of superiority of either of the two glycometrics when evaluating the psychosocial sphere of T1D adult.
So far, only one Italian group [10] has evaluated the satisfaction of an exclusively pediatric cohort and its relationship with GRI using a CGM satisfaction questionnaire (CGM-SAT). This cross-sectional study shows how satisfaction with CGM was significantly related to TIR and negatively related to GRI, in line with what was observed in our study. However, Marigliano’s study uses questionnaires specifically aimed at assessing satisfaction with a given CGM system in a pediatric population (with the difficulties inherent to the assessment of satisfaction in childhood). Moreover, it does not use specific scales to assess diabetes-specific quality of life, disease stress, global quality of life, degree of knowledge or perception of validated hypoglycemia.
Given the close correlation between TIR and GRI [17, 18], our results are partly to be expected. However, the weighting of CHypo in GRI calculation [14] due to its clinical importance could theoretically lead us to expect a better correlation of quality of life with GRI. Our study failed to demonstrate a superiority of the GRI when assessing the degree of quality of life, nor correlation with the degree of diabetes-related stress or quality of life with TBR or TAR. But it supports the individual evaluation of both components of Hyper and Hypoglycemia. In fact, CHyper and CHypo were more strongly associated with worse scores in DQoL, VAS, and DDS; and to a lower degree of knowledge (DKQ2). In the multiple linear regression model to predict DQoL, only CHyper and age maintained statistical significance, not CHypo, despite being the most penalized factor in the GRI calculation, and other variables such as education level showed no difference. It may be due to two factors: Although the patients evaluated were randomly selected and those with unawareness hypoglycemia were not excluded, the degree of overall hypoglycemia in our population is not high (TBR 54–70 4.3 (2.8)). In this sense, the T1D patients evaluated could have related their quality of life more to the CHyper than to the risk of hypoglycemia. Not surprisingly, the highest correlation among all those glycometric evaluated was between CHyper with DQoL (r = 0.422; p < 0.05). Furthermore, the fact that we found no differences in GRI and its components between patients with a positive or negative Clarke’s test supports the thesis of an underrepresentation of patients with undetected hypoglycemia, a subgroup that would penalize quality of life in a greater way in relation to CHypo. Nevertheless, our results are congruent with the Marigliano et al. study where no significant relationships were observed between satisfaction with CGM and glycometric such as TBR, TAR or CV [10]. Furthermore, the positive results in the psychosocial sphere in those subjects with better glycemic control by CHyper could be related to a greater awareness of the disease, as well as a greater use of therapeutic education programs, which would be reflected in a higher degree of knowledge in the group with CHyper < 15.
Furthermore, although no differences were observed between the GRI groups (A–E) and the different questionnaires, there does seem to be a tendency in those patients with better GRI (Groups A and B) towards greater satisfaction with treatment and better quality of life, with the stress level being higher in the more extreme groups (A, D and E). In group A, it could be related to a greater self-demand/burden related to the disease for optimal control of their diabetes.
Our study has certain limitations. First, it is a single-center cross-sectional study with a relatively small sample size compared to big data studies; however, it is a real-life cohort with stable control, with different types of treatment (MDI and CSII) and comprehensive knowledge of glycometric and clinical variables with a single current CGM system where quality of life, knowledge and degree of diabetes-related stress have been comprehensively assessed in a systematic and representative manner. Furthermore, our results are in line with the few studies published to date on quality of life and TIR [5, 12] and on the relationship between TIR and GRI [14, 17, 18]. Our study also has strengths, this the first to relate GRI and its components in T1D to quality of life, diabetes-related stress, perception of hypoglycemia, satisfaction with treatment, and degree of knowledge. In addition, it sheds light on new glycometric parameters that may be important in the future when assessing quality of life in subjects with diabetes. In this sense, it is important to mention that the weak correlation found between the different variables evaluated is not due to low statistical power but to the difficulty of finding significant relationships in a complex sphere as the psychosocial [12]. Further longitudinal evaluation studies and studies in specific populations (especially those at high risk of hypoglycemia or unawareness), as well as in other types of diabetes, are necessary to demonstrate the present results.
In conclusion, the GRI correlated with improved outcomes in quality of life, diabetes-related stress, and satisfaction with treatment, with no differences in the level of knowledge or perception of hypoglycemia, analogous to the TIR. The parameters that were related to a greater decline in quality of life, diabetes-related stress, and lower satisfaction with treatment were CHyper and Chypo. However, in a multiple linear regression, only CHyper maintained statistical significance.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Valladolid University.
Abbreviations
- GRI:
-
glycemia risk index
- CHypo:
-
component of hypoglycemia
- CHyper:
-
component of hyperglycemia
- DQoL:
-
diabetes quality of life questionnaire
- DDS:
-
diabetes-related distress scale
- VAS:
-
visual analogic scale (VAS)
- DKQ2:
-
diabetes knowledge questionnaire
- TIR:
-
time in range (70–180 mg/dl)
- TBR:
-
time below Range (<70 mg/dl)
- TAR:
-
time above range (>180 mg/dL)
- T1D Type:
-
1 diabetes
- isCGM:
-
flash glucose monitoring or intermittent scanned continuous glucose monitoring
- CGM:
-
continous glucose monitoring
- AGP:
-
ambulatory glucose profile
- HbA1c:
-
glycated hemoglobin
- MDI:
-
multiple doses of insulin
- CSII:
-
continuous subcutaneous insulin infusion
- Pc:
-
percentiles
- GMI:
-
glucose management indicator (GMI)
- CV:
-
coefficient of variation
- TBR<54:
-
time below 54 mg/dL
- TBR 54–70:
-
time between 54 and 70 mg/dL in Range
- TAR 180–250:
-
time between 81 and 250 mg/dL
- TAR>250:
-
time above 250 mg/dL
References
T. Danne, R. Nimri, T. Battelino, R.M. Bergenstal, K.L. Close, J.H. DeVries, et al., International consensus on use of continuous glucose monitoring. Diabetes Care 40(12), 1631–1640 (2017)
T. Battelino, T. Danne, R.M. Bergenstal, S.A. Amiel, R. Beck, T. Biester, et al., Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42(8), 1593–1603 (2019)
T. Battelino, C.M. Alexander, S.A. Amiel, G. Arreaza-Rubin, R.W. Beck, R.M. Bergenstal, et al., Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol. 11(1), 42–57 (2023)
E. Ang, Z.X. Lee, S. Moore, M. Nana, Flash glucose monitoring (FGM): a clinical review on glycaemic outcomes and impact on quality of life. J. Diabetes Complicat. 34(6), 107559 (2020)
F. Gomez-Peralta, T. Dunn, K. Landuyt, Y. Xu, J.F. Merino-Torres, Flash glucose monitoring reduces glycemic variability and hypoglycemia: real-world data from Spain. BMJ Open Diabetes Res. Care 8(1), e001052 (2020)
G. Kramer, L. Michalak, U.A. Müller, C. Kloos, C. Werner, N. Kuniss, Association between flash glucose monitoring and metabolic control as well as treatment satisfaction in outpatients with diabetes type 1. Exp. Clin. Endocrinol. Diabetes 129(4), 303–308 (2021)
P. Oskarsson, R. Antuna, P. Geelhoed-Duijvestijn, J. Krӧger, R. Weitgasser, J. Bolinder, Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia 61(3), 539–550 (2018)
S. Mitsuishi, R. Nishimura, S.I. Harashima, T. Kawamura, D. Tsujino, K. Koide, et al., The effect of novel glucose monitoring system (flash glucose monitoring) on mental well-being and treatment satisfaction in Japanese people with diabetes. Adv .Ther. 35(1), 72–80 (2018)
G. Díaz-Soto, M.P. Bahíllo-Curieses, R. Jimenez, M. Nieto, O. de la, E. Gomez, B. Torres, et al., The relationship between glycosylated hemoglobin, time-in-range and glycemic variability in type 1 diabetes patients under flash glucose monitoring. Endocrinol. Diabetes Nutr. 68(7), 465–471 (2021)
M. Marigliano, R. Pertile, E. Mozzillo, A. Troncone, C. Maffeis, E. Morotti, et al., Satisfaction with continuous glucose monitoring is positively correlated with time in range in children with type 1 diabetes. Diabetes Res. Clin. Pract. 204, 110895 (2023)
The DCCT Research GroupReliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care 11(9), 725–732 (1988)
J. Speight, P. Choudhary, E.G. Wilmot, C. Hendrieckx, H. Forde, W.Y. Cheung, et al., Impact of glycaemic technologies on quality of life and related outcomes in adults with type 1 diabetes: a narrative review. Diabet. Med. 40(1), e14944 (2023)
E.C. Cobry, L.G. Kanapka, E. Cengiz, L. Carria, L. Ekhlaspour, B.A. Buckingham, et al., Health-related quality of life and treatment satisfaction in parents and children with type 1 diabetes using closed-loop control. Diabetes Technol. Ther. 23(6), 401–409 (2021)
D.C. Klonoff, J. Wang, D. Rodbard, M.A. Kohn, C. Li, D. Liepmann, et al., A glycemia risk index (GRI) of hypoglycemia and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J. Diabetes Sci. Technol. 17(5), 1226–1242 (2023)
G. Díaz-Soto, P. Pérez-López, P. Férnandez-Velasco, M.O. Nieto de la Marca, E. Delgado, S. Del Amo, et al., Glycemia risk index assessment in a pediatric and adult patient cohort with type 1 diabetes mellitus. J. Diabetes Sci. Technol. 16:19322968231154561 (2023). Epub ahead of print
P.Y. Benhamou, A. Adenis, Y. Tourki, S. Pou, S. Madrolle, S. Franc, et al., Efficacy of a hybrid closed-loop solution in patients with excessive time in hypoglycaemia: a post hoc analysis of trials with DBLG1 system. J. Diabetes Sci. Technol. 18(2), 372–379 (2024)
J.Y. Kim, J.H. Yoo, J.H. Kim, Comparison of glycemic risk index (GRI) with time in range for assessing glycemic quality. Diabetes Technol. Ther. 25(12), 883–892 (2023)
P. Pérez-López, P. Férnandez-Velasco, P. Bahillo-Curieses, D. de Luis, G. Díaz-Soto, Impact of glucose variability on the assessment of the glycemia risk index (GRI) and classic glycemic metrics. Endocrine 82(3), 560–568 (2023)
V.N. Shah, C. Sakamoto, L. Pyle, Optimal sampling duration for continuous glucose monitoring for the estimation of glycemia risk index. Diabetes Technol. Ther. 25(2), 140–142 (2023)
W.H. Polonsky, L. Fisher, J. Earles, R.J. Dudl, J. Lees, J. Mullan, et al., Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 28(3), 626–631 (2005)
W.L. Clarke, D.J. Cox, L.A. Gonder-Frederick, D. Julian, D. Schlundt, W. Polonsky, Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 18(4), 517–522 (1995)
A.A. Garcia, E.T. Villagomez, S.A. Brown, K. Kouzekanani, C.L. Hanis, The Starr County Diabetes Education Study: development of the Spanish-language diabetes knowledge questionnaire. Diabetes Care 24(1), 16–21 (2001)
J. Lu, X. Ma, J. Zhou, L. Zhang, Y. Mo, L. Ying, et al., Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 41(11), 2370–2376 (2018)
J.H. Yoo, J.Y. Kim, J.H. Kim, Association between continuous glucose monitoring-derived glycemia risk index and albuminuria in type 2 diabetes. Diabetes Technol. Ther. 25(10), 726–735 (2023)
R. Jiménez-Sahagún, E. Gómez Hoyos, G. Díaz Soto, M. Nieto de la Marca, O. de la, M. Sánchez Ibáñez, S. Del Amo Simón, et al., Impact of flash glucose monitoring on quality of life and glycaemic control parameters in adults with type 1 diabetes mellitus⋆. Endocrinol. Diabetes Nutr. 69(5), 345–353 (2022)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Gonzalo Díaz Soto and Paloma Pérez López, investigation, writing—original draft, supervision, writing–review & editing, Pablo Férnandez Velasco, investigation; Pilar Bahillo, investigation; Rebeca Jimenez, investigation; María de la O Nieto de la Marca, investigation, Daniel de Luis, review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Díaz-Soto, G., Pérez-López, P., Férnandez-Velasco, P. et al. Quality of life, diabetes-related stress and treatment satisfaction are correlated with glycemia risk index (GRI), time in range and hypoglycemia/hyperglycemia components in type 1 diabetes. Endocrine (2024). https://doi.org/10.1007/s12020-024-03846-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03846-9