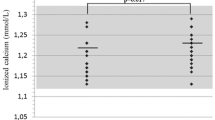
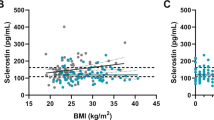
Abstract
Purpose
Patients receiving long-term glucocorticoid (GC) treatment are at risk of osteoporosis, while bone effects of substitution doses in Addison’s disease (AD) remain equivocal. The project was aimed to evaluate serum bone turnover markers (BTMs): osteocalcin, type I procollagen N-terminal propeptide (PINP), collagen C-terminal telopeptide (CTX), sclerostin, DKK-1 protein, and alkaline phosphatase (ALP) in relation to bone mineral density (BMD) during GC replacement.
Methods
Serum BTMs and hormones were assessed in 80 patients with AD (22 males, 25 pre- and 33 postmenopausal females) on hydrocortisone (HC) substitution for ≥3 years. Densitometry with dual-energy X-ray absorptiometry covered the lumbar spine (LS) and femoral neck (FN).
Results
Among BTMs, only PINP levels were altered in AD. BMD Z-scores remained negative except for FN in males. Considering T-scores, osteopenia was found in LS in 45.5% males, 24% young and 42.4% postmenopausal females, while osteoporosis in 9.0%, 4.0% and 21.1%, respectively. Lumbar BMD correlated positively with body mass (p = 0.0001) and serum DHEA-S (p = 9.899 × 10−6). Negative correlation was detected with HC dose/day/kg (p = 0.0320), cumulative HC dose (p = 0.0030), patient’s age (p = 1.038 × 10−5), disease duration (p = 0.0004), ALP activity (p = 0.0041) and CTX level (p = 0.0105). However, only age, body mass, ALP, serum CTX, and sclerostin remained independent predictors of LS BMD.
Conclusion
Standard HC substitution does not considerably accelerate BMD loss in AD patients and their serum BTMs: CTX, osteocalcin, sclerostin, DKK-1, and ALP activity remain within the reference ranges. Independent predictors of low lumbar spine BMD, especially ALP activity, serum CTX and sclerostin, might be monitored during GC substitution.
Similar content being viewed by others
References
E.S. Husebye, B. Allolio, W. Arlt et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J. Intern. Med. 275(2), 104–115 (2014). https://doi.org/10.1111/joim.12162
G. Johannsson, A. Falorni, S. Skrtic et al. Adrenal insufficiency: review of clinical outcomes with current glucocorticoid replacement therapy. Clin. Endocrinol. (Oxf) 82(1), 2–11 (2015). https://doi.org/10.1111/cen.12603
V. Guarnotta, C. Di Stefano, C. Giordano, Long-term outcomes of conventional and novel steroid replacement therapy on bone health in primary adrenal insufficiency. Sci. Rep. 12(1), 13280 (2022). https://doi.org/10.1038/s41598-022-13506-5
K. Løvås, C.G. Gjesdal, M. Christensen et al. Glucocorticoid replacement therapy and pharmacogenetics in Addison’s disease: effects on bone. Eur. J. Endocrinol. 160(6), 993–1002 (2009). https://doi.org/10.1530/EJE-08-0880
M. Yazidi, C. Danguir, D. Maamer et al. Impact of hydrocortisone replacement on bone mineral density and bone turnover markers in patients with primary adrenal insufficiency. Endocr. Regul. 56(3), 209–215 (2022). https://doi.org/10.2478/enr-2022-0022
L. Li, S. Bensing, H. Falhammar, Rate of fracture in patients with glucocorticoid replacement therapy: a systematic review and meta-analysis. Endocrine 74, 29–37 (2021). https://doi.org/10.1007/s12020-021-02723-z
J. Compston, Glucocorticoid-induced osteoporosis: an update. Endocrine 61(1), 7–16 (2018). https://doi.org/10.1007/s12020-018-1588-2
Z.E. Belaya, T.A. Grebennikova, G.A. Melnichenko et al. Effects of endogenous hypercortisolism on bone mRNA and microRNA expression in humans. Osteoporos. Int. 29(1), 211–221 (2018). https://doi.org/10.1007/s00198-017-4241-7
T. van Staa, H.G. Leufkens, C. Cooper, The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos. Int. 13(10), 777–787 (2002). https://doi.org/10.1007/s001980200108
K.R. Koetz, M. Ventz, S. Diederich, M. Quinkler, Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J. Clin. Endocrinol. Metab. 97(1), 85–92 (2012). https://doi.org/10.1210/jc.2011-2036
D.D. Chandy, E. Bhatia, Bone mineral density in patients with Addison’s disease on replacement therapy with prednisolone. Endocr. Pract. 22(4), 434–439 (2016). https://doi.org/10.4158/EP151014.OR
K.R. Frey, T. Kienitz, J. Schulz et al. Prednisolone is associated with a worse bone mineral density in primary adrenal insufficiency. Endocr. Connect. 7(6), 811–818 (2018). https://doi.org/10.1530/EC-18-0160
N. Esteban, T. Loughlin, A.L. Yergey et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J. Clin. Endocrinol. Metab. 72(1), 39–45 (1991). https://doi.org/10.1210/jcem-72-1-39
W. Arlt, C. Rosenthal, S. Hahner, B. Allolio, Quality of glucocorticoid replacement in adrenal insufficiency: clinical assessment vs. timed serum cortisol measurements. Clin. Endocrinol. (Oxf) 64(4), 384–389 (2006). https://doi.org/10.1111/j.1365-2265.2006.02473.x
M. Fichna, M. Gryczyńska, A. Sowińska, J.Sowiński, Ocena metaboliczna terapii substytucyjnej hydrokortyzonem u pacjentów z pierwotna niedoczynnościa kory nadnerczy [Metabolic assessment of hydrocortisone replacement therapy in patients with primary adrenocortical insufficiency]. Przegl. Lek. 68(2), 96–102 (2011)
V. Camozzi, C. Betterle, A.C. Frigo et al. Vertebral fractures assessed with dual-energy X-ray absorptiometry in patients with Addison’s disease on glucocorticoid and mineralocorticoid replacement therapy. Endocrine 59(2), 319–329 (2018). https://doi.org/10.1007/s12020-017-1380-8
F. Heureux, D. Maiter, Y. Boutsen, J.P. Devogelaer, J. Jamart, J. Donckier, Evaluation de la substitution par corticostéroïdes et de ses répercussions osseuses dans la maladie d’Addison [Evaluation of corticosteroid replacement therapy and its effect on bones in Addison’s disease]. Ann. Endocrinol. (Paris) 61(3), 179–183 (2000)
S. Kaptoge, L.I. Benevolenskaya, A.K. Bhalla et al. Low BMD is less predictive than reported falls for future limb fractures in women across Europe: results from the European Prospective Osteoporosis Study. Bone 36(3), 387–398 (2005). https://doi.org/10.1016/j.bone.2004.11.012
P.D. Delmas, R. Eastell, P. Garnero, M.J. Seibel, J. Stepan; Committee of Scientific Advisors of the International Osteoporosis Foundation, The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos. Int. 6, S2–S17 (2000). https://doi.org/10.1007/s001980070002
S. Vasikaran, R. Eastell, O. Bruyère et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos. Int. 22(2), 391–420 (2011). https://doi.org/10.1007/s00198-010-1501-1
J. Delgado-Calle, A.Y. Sato, T. Bellido, Role and mechanism of action of sclerostin in bone. Bone 96, 29–37 (2017). https://doi.org/10.1016/j.bone.2016.10.007
P. Garnero, E. Sornay-Rendu, B. Claustrat, P.D. Delmas, Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J. Bone Miner. Res. 15(8), 1526–1536 (2000). https://doi.org/10.1359/jbmr.2000.15.8.1526
L. Gifre, S. Ruiz-Gaspà, A. Monegal et al. Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone 57(1), 272–276 (2013). https://doi.org/10.1016/j.bone.2013.08.016
A.L. Burshell, R. Möricke, R. Correa-Rotter et al. Correlations between biochemical markers of bone turnover and bone density responses in patients with glucocorticoid-induced osteoporosis treated with teriparatide or alendronate. Bone 46(4), 935–939 (2010). https://doi.org/10.1016/j.bone.2009.12.032
M. Tóth, A. Grossman, A. Glucocorticoid-induced osteoporosis: lessons from Cushing’s syndrome. Clin. Endocrinol. (Oxf) 79(1), 1–11 (2013). https://doi.org/10.1111/cen.12189
M.A. Valero, M. Leon, M.P. Ruiz Valdepeñas et al. Bone density and turnover in Addison’s disease: effect of glucocorticoid treatment. Bone Miner 26(1), 9–17 (1994). https://doi.org/10.1016/s0169-6009(08)80158-4
S.R. Peacey, C.Y. Guo, A.M. Robinson et al. Glucocorticoid replacement therapy: are patients over treated and does it matter? Clin. Endocrinol. (Oxf) 46(3), 255–261 (1997). https://doi.org/10.1046/j.1365-2265.1997.780907.x
M. Wichers, W. Springer, F. Bidlingmaier, D. Klingmüller, The influence of hydrocortisone substitution on the quality of life and parameters of bone metabolism in patients with secondary hypocortisolism. Clin. Endocrinol. (Oxf) 50(6), 759–765 (1999). https://doi.org/10.1046/j.1365-2265.1999.00723.x
A. Zdrojowy-Wełna, J. Halupczok-Żyła, N. Słoka, J. Syrycka, Ł. Gojny, M. Bolanowski, Bolanowski Trabecular bone score and sclerostin concentrations in patients with primary adrenal insufficiency. Front. Endocrinol. (Lausanne) 13, 996157 (2022). https://doi.org/10.3389/fendo.2022.996157
J.A. Kanis, L.J. Melton 3rd, C. Christiansen, C.C. Johnston, N. Khaltaev, The diagnosis of osteoporosis. J. Bone. Miner. Res. 9(8), 1137–1141 (1994). https://doi.org/10.1002/jbmr.5650090802
P. Głuszko, E. Sewerynek, W. Misiorowski et al. Guidelines for the diagnosis and management of osteoporosis in Poland. Update 2022. Endokrynol. Pol. 74(1), 5–15 (2023). https://doi.org/10.5603/EP.a2023.0012
M. Penna-Martinez, G. Meyer, A.B. Wolff et al. Vitamin D status and pathway genes in five European autoimmune Addison’s disease cohorts. Eur. J. Endocrinol. 184(3), 373–381 (2021). https://doi.org/10.1530/EJE-20-0956
W. Guo, F. Li, C. Zhu et al. Effect of hypercortisolism on bone mineral density and bone metabolism: A potential protective effect of adrenocorticotropic hormone in patients with Cushing’s disease. J. Int. Med. Res. 46(1), 492–503 (2018). https://doi.org/10.1177/0300060517725660
F.N. Ton, S.C. Gunawardene, H. Lee, R.M. Neer, Effects of low-dose prednisone on bone metabolism. J. Bone Miner. Res. 20(3), 464–470 (2005). https://doi.org/10.1359/JBMR.041125
A.M. Suliman, R. Freaney, T.P. Smith, Y. McBrinn, B. Murray, T.J. McKenna, The impact of different glucocorticoid replacement schedules on bone turnover and insulin sensitivity in patients with adrenal insufficiency. Clin. Endocrinol. (Oxf) 59(3), 380–387 (2003). https://doi.org/10.1046/j.1365-2265.2003.01860.x
J.T. Schousboe, J.A. Shepherd, J.P. Bilezikian, S. Baim, Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J. Clin. Densitom. 16(4), 455–466 (2013). https://doi.org/10.1016/j.jocd.2013.08.004
J.P. Devogelaer, J. Crabbé, C. Nagant de Deuxchaisnes, Bone mineral density in Addison’s disease: evidence for an effect of adrenal androgens on bone mass. Br. Med. J. (Clin Res Ed 294(6575), 798–800 (1987). https://doi.org/10.1136/bmj.294.6575.798
G.D. Braatvedt, M. Joyce, M. Evans, J. Clearwater, I.R. Reid, Bone mineral density in patients with treated Addison’s disease. Osteoporos. Int. 10(6), 435–440 (1999). https://doi.org/10.1007/s001980050251
E.M. Gurnell, P.J. Hunt, S.E. Curran et al. Long-term DHEA replacement in primary adrenal insufficiency: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 93(2), 400–409 (2008). https://doi.org/10.1210/jc.2007-1134
K. Mukaiyama, M. Kamimura, S. Uchiyama, S. Ikegami, Y. Nakamura, H. Kato, Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin. Exp. Res. 27(4), 413–418 (2015). https://doi.org/10.1007/s40520-014-0296-x
A.H. van Lierop, A.W. van der Eerden, N.A. Hamdy, A.R. Hermus, M. den Heijer, S.E. Papapoulos, Circulating sclerostin levels are decreased in patients with endogenous hypercortisolism and increase after treatment. J. Clin. Endocrinol. Metab. 97(10), E1953–E1957 (2012). https://doi.org/10.1210/jc.2012-2218
C. Fernández-Roldán, F. Genre, R. López-Mejías et al. Sclerostin serum levels in patients with systemic autoimmune diseases. Bonekey Rep. 5, 775 (2016). https://doi.org/10.1038/bonekey.2016.2
E. Shevroja, J.Y. Reginster, O. Lamy et al. Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging. Osteoporos. Int. 34(9), 1501–1529 (2023). https://doi.org/10.1007/s00198-023-06817-4
F. Bioletto, M. Barale, M. Parasiliti-Caprino et al. Bone safety of dual-release hydrocortisone in patients with autoimmune primary adrenal insufficiency. Front. Endocrinol (Lausanne). 11(14), 1234237 (2023). https://doi.org/10.3389/fendo.2023.1234237
Acknowledgements
We express our gratitude to all our patients with Addison’s disease for their participation and comprehension.
Author contributions
All authors contributed to the study conception. K.F., P.G. and M.F. were involved in study design. Material preparation was performed by P.G., M.R. and M.F. Data collection and analysis was done by K.F., A.S. and M.F. The first draft of the manuscript was written by K.F. and all authors commented and provided feedback to the final version. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was performed in accordance with the principles of the Declaration of Helsinki. Its protocol was approved by the local Ethics Committee of Poznan University of Medical Sciences (decision 68/19). Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Furman, K., Gut, P., Sowińska, A. et al. Predictors of bone mineral density in patients receiving glucocorticoid replacement for Addison’s disease. Endocrine 84, 711–719 (2024). https://doi.org/10.1007/s12020-024-03709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-024-03709-3