Abstract
Purpose
Continuous Glucose Monitoring (CGM) is a key tool for insulin-treated people with diabetes (PwD). CGM devices include both real-time CGM (rtCGM) and intermittently scanned CGM (isCGM), which are associated with an improvement of glucose control and less hypoglycemia in clinical trials of people with type 1 and type 2 diabetes.
Methods
This is an expert position to update a previous algorithm on the most suitable choice of CGM for insulin-treated PwD in light of the recent evidence and clinical practice.
Results
We identified six different clinical scenarios, including type 1 diabetes, type 2 diabetes, pregnancy on intensive insulin therapy, regular physical exercise, new onset of diabetes, and frailty. The use of rtCGM or isCGM is suggested, on the basis of the predominant clinical issue, as suboptimal glucose control or disabling hypoglycemia, regardless of baseline HbA1c or individualized HbA1c target.
Conclusion
The present algorithm may help to select the best CGM device based on patients’ clinical characteristics, needs and clinical context, offering a further opportunity of a “tailored” therapy for people with insulin-treated diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Continuous Glucose Monitoring (CGM) is a key tool for insulin-treated people with diabetes (PwD) [1, 2] measuring interstitial glucose level on a near-continuous basis, and providing information about glucose trend and rate of change. When appropriately used, CGM helps PwD make therapeutic decision before meals, exercise and any other condition that may influence glucose levels. A recent meta-analysis demonstrated that PwD, both type 1 and type 2, using CGM systems experience fewer hyperglycemic and hypoglycemic episodes, with a modest but significant decrease in glycated hemoglobin (HbA1C) levels [3].
A typical CGM requires three elements, the sensor, the transmitter, and the receiver. The sensor is placed under the skin and sends glucose readings to the transmitter attached to the skin above the sensor. The transmitter wirelessly sends glucose measurements to the receiver, which is a dedicated device or smart device (smartphone and smartwatch) by an App. Current available CGM devices include both real-time CGM (rtCGM), which continuously provides current and predicted glucose levels, and intermittently scanned CGM (isCGM), which displays glucose values when the transmitter is swiped by a reader or a smartphone. Moreover, a third type of CGM, the professional or “blinded” CGM is used by health care professionals as a diagnostic tool to retrospectively evaluate glycemic patterns and trends. CGM systems have distinctive characteristics, sensor lifetime (from 7 days to 6 months), accuracy, smartphone compatibility, insulin pump integration, costs, alarms and alerts, and user-friendliness. Furthermore, the systems may require different calibration conditions, have an adjunctive or non-adjunctive use, and can be integrated with insulin pump. Finally, the glucose measurements are processed by different software which summarize and display the data according to the international consensus on time in range and AGP (Ambulatory Glucose Profile) consensus [4]. (Table 1). The choice of a CGM system will depend on patient needs and preferences, system features, patient characteristics including the type of diabetes, individual skills, glucose control, concomitant morbidities, frailty, pregnancy, and cost.
CGM is strongly recommended for PwD treated with intensive insulin therapy. Both international [2, 5] and Italian [6] guidelines highlight the importance of selecting and educating patients and caregivers to the use of CGM. The guidelines identify poor glucose control and hypoglycemia as the main indications for CGM use, especially in case of problematic hypoglycemia (frequent, severe, nocturnal, not perceived).
We aimed our current expert position to update the prior published algorithm on the most suitable choice of CGM for insulin-treated PwD in light of the recent evidence reporting the efficacy and safety of CGM in type 1 and type 2 diabetes [7,8,9], and clinical practice.
The updated algorithm
The previous algorithm [10] has been extensively revised in order to support the use of rtCGM and isCGM in different clinical scenarios. Our suggestions based on evidence and good clinical practice will allow a more comprehensive approach, tailoring possible CGM solutions according to the clinical issues and needs of PwD.
The structured self-monitoring blood glucose (SMBG) (at least four fingerpicks per day) together with reinforced patient-centered education remains the first-line glucose monitoring strategy for adults with diabetes on intensive insulin therapy [2].
The second-line strategy with rtCGM or isCGM is suggested, regardless of baseline HbA1c or individualized HbA1c target, on the basis of the predominant clinical issue, as suboptimal glucose control or disabling hypoglycemia.
Six different clinical scenarios have been identified: type 1 diabetes, type 2 diabetes, pregnancy on intensive insulin therapy, regular physical exercise, new onset of diabetes, and frailty. The choice of CGM system is based on two main factors, the ‘suboptimal glucose control’ and risk of ‘disabling hypoglycemia’. The suboptimal glucose control is referred to HbA1c not at desired target, and disabling hypoglycemia to history of frequent severe hypoglycemia, nocturnal hypoglycemia, and hypoglycemia unawareness. Additional CGM features are also taken into account for each scenario, as reported below.
First scenario: type 1 diabetes
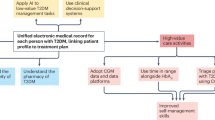
Figure 1. In people with type 1 diabetes with disabling hypoglycemia, or previous episodes of diabetic ketoacidosis (DKA), we suggest the rtCGM to customize alerts and alarms in order to predict impending hypoglycemia, acting quickly and avoid a potentially serious event. Systems with high accuracy in the low glucose range are recommended (Table 3). In the case of suboptimal glucose control, isCGM and rtCGM can both be suggested, depending on individual skills, patient preferences, smart devices availability, and costs.
CGM represents a fundamental therapeutic tool in type 1 diabetes since it allows to address the potential issues related to the "timing" of insulin administered at meals, the insulin-carbohydrate ratio (and more generally, the influence of other nutrients including proteins and fats) and the management of night period. In RCTs of adults with type 1 diabetes, both isCGM and rtCGM are effective in increasing the TIR and reducing the time spent in hypoglycemia (time below range, TBR) [7, 8, 11,12,13]. Clinical benefits in terms of improved glucose control have been reported for youth and middle aged adults with poor diabetes control (HbA1c ranging between 7.5%–11.0%) using rtCGM, and middle aged adults with HbA1c closer to optimal range (6.8–8.7%) using isCGM (Table 2). Specifically, rtCGM with predictive alerts and alarms was associated with a reduction of a number of hypoglycemic events [14], including severe hypoglycemia [15, 16], decrease in TBR [15,16,17,18,19], and an improvement in hypoglycemia awareness [15, 16, 20] in middle aged adults with type 1 diabetes prone to hypoglycemia or impaired awareness of hypoglycemia, and HbA1c ranging between 7.5% and 8.2% (Table 2). Since hypoglycemia is the limiting factor of diabetes therapy and a major concerns for people with diabetes and their families, reducing both level 1 and level 2 hypoglycemia is critical to improve glucose daily oscillations, quality of life and diabetes distress and prevent serious hypoglycemic episodes occurring with loss of consciousness or seizures, respectively. Moreover, clinicians should be aware of the accuracy of the used device, in order to choice the systems with high accuracy in presence of persistent of prolonged hypoglycemia (Table 3).
Second scenario: diabetes onset
The isCGM should be proposed in each patient at the onset of diabetes when insulin therapy is required (Fig. 2). Its use should be also considered in people with a recent diagnosis of diabetes presenting prevalent hyperglycemia or in people with autoimmune diabetes in the stage of “honeymoon” with reduced insulin requirements. In the presence of frequent and severe hypoglycemia (with or without recognized precipitating cause) and poor glucose control despite intensive insulin therapy, rtCGM may be used due to the possibility of customizable alerts/alarms that allow patients to recognize in advance the effect of actions/factors that influence glucose variability. In people who are unwilling or unable to engage in the routine use of CGM, the occasional use of isCGM may be suggested to evaluate glucose trends and to guide the related therapeutic choices.
Preventing diabetic complications and optimizing the quality of life are both goals of diabetes therapy. A person-centered approach, tailored on both individual preferences and needs, is essential for effective diabetes management [21]. Therefore, the achievement of glucose control should be pursued from the early stages to preserve PwD from long-term disabling complications [21, 22]. Expanding the use of CGM to all people at diabetes onset needing insulin therapy may support effective intensification of treatments valuable to reduce glucose exposure and glucose variability and lower the risk of complications and hospital admissions, which are associated with high economic burden. Of note, these goals may be achieved while minimizing the risk of hypoglycemia and improving quality of life for people with diabetes. Moreover, the analysis of glucose pattern may be used as an educational tool to demonstrate the relationship between an individual’s glucose levels, his/her medication, and other therapeutic interventions. There is evidence from observational studies that the early use (within one year from the diagnosis) of both isCGM [23] and rtCGM [24,25,26] results in long-term improvement in HbA1c and the reduction in diabetes-related emergency-department admissions in people with type 1 diabetes. Moreover, the use of CGM at the onset of diabetes allows patients to be aware of the glycemic trends and their changes following insulin therapy. On the other hand, CGM may help physicians to monitor glycemic control and hence optimize structured therapeutic education remotely. The use of an isCGM as the first choice at diabetes onset may be preferred, as it provides a less expensive device with automatic transmission of data, does not need calibrations, and is approved for insulin dosing without SMBG. Only when symptoms of hypoglycemia do not match the glucose level detected, or glucose levels are rapidly changing, SMBG has to be considered. Moreover, isCGM with optional alarms for high and low blood glucose may be useful in patients in good metabolic control at low risk of hypoglycemia who have physical, psychological, or occupational barriers to the regular use of capillary glucose monitoring. A rtCGM should be reserved to people with a recent diabetes diagnosis and poor glucose control (i.e. high glucose variability) or disabling hypoglycemia: this should be based on the possibility to set alarms for hypoglycemia, hyperglycemia and rapid variations in glucose, increasing further the effectiveness of clinical management.
Third scenario: type 2 diabetes
Figure 3. In patients with type 2 diabetes treated with insulin therapy in whom hypoglycemia predominates, a rtCGM with predictive alerts and alarms should be used. In type 2 PwD with suboptimal glucose control and low risk for hypoglycemia who require more data than those offered by SMBG, both isCGM and rtCGM can be suggested, depending on the individual’s circumstances, preferences, and needs.
Studies evaluating the effects of CGM in people with type 2 diabetes are limited, especially those including patients not on intensive insulin therapy [9]. There is evidence from RCTs that rtCGM reduces HbA1c and increases TIR in people with type 2 diabetes treated with multiple daily injections [27] or basal insulin [9], and is more effective than SMBG in minimizing hypoglycemia for people using therapies at high hypoglycemic risk [28]. Moreover, a recent large retrospective cohort study including 41753 participants with insulin-treated diabetes, of whom 36 080 with type 2 diabetes, showed that among adults who started rtCGM, those with type 2 diabetes had greater improvements in HbA1c than patients with type 1 diabetes associated with reductions in emergency department visits and hospitalizations for hypoglycemia [29]. Of note, recent practice guidelines suggest the use of rtCGM for people with type 2 diabetes who take insulin and are at risk for hypoglycemia [30]. Furthermore, the intermittent use of rtCGM in people with type 2 diabetes on oral anti-diabetic drugs, with or without basal insulin, is emerging as an effective strategy in lowering HbA1c in the short-term [31, 32].
In a recent meta-analysis of RCTs, the use of isCGM was shown to significantly reduce HbA1c levels of −0.74% in participants with T2D, with a lower risk of hypoglycemia compared with SMBG, and a greater effect in participants aged ≤ 65 years [33]. A significant improvement in HbA1c associated with isCGM has also been found in an observational study of people with type 2 diabetes on basal-bolus therapy [34]. Evidence relative to the efficacy of isCGM in improving glycemic outcomes, including a greater TIR, a lower TBR, and a higher reduction in HbA1c levels, are also emerging among non-insulin-treated people with type 2 diabetes [35]. Finally, the use of isCGM should be a valuable therapeutic opportunity in people with type 2 diabetes and fair glucose control [36].
Fourth scenario: diabetes and pregnancy on intensive insulin therapy
Figure 4. In the presence of pre-gestational diabetes (both type 1 and type 2), or gestational diabetes on intensive insulin therapy and suboptimal glucose control, the preferential use of rtCGM or alternatively an isCGM should be considered in addition to SMBG in order to frequently monitor blood glucose levels and achieve glucose targets throughout pregnancy. If there is a risk of maternal hypoglycemia, a rtCGM should be preferred.
An increasing corpus of evidence demonstrates that CGM (mainly rtCGM) can improve maternal pre-natal glucose levels and neonatal outcomes in women with pre-existing diabetes or gestational diabetes [37, 38]. The critical issues traditionally associated with insulin therapy in the management of diabetes become more evident during pregnancy, based on the reduced insulin sensitivity and tighter fasting and postprandial blood glucose targets to be achieved for the prevention of maternal and neonatal complications. In addition, the International Consensus on TIR endorses ambitious glucose targets for pregnant women with pre-existing type 1 diabetes [TIR 63–140 mg/dl (> 70%), TAR > 140 mg/dl (< 25%), TBR < 63 mg/dl (< 4%), TBR < 54 mg/dl (< 1%)] or pre-existing type 2 diabetes and gestational diabetes [4]. Therefore, the use of rtCGM over isCGM should be preferred because of the accuracy of the data, the possibility of enable high and low glucose alerts and predictive alarms for hypoglycemia, allowing an intense pattern of care while minimizing the risk of maternal hypoglycemia.
Fifth scenario: regular physical exercise
Figure 5 Both rtCGM and isCGM should be suggested in PwD performing sporting activities, based on individual preferences and personal experiences. In case of disabling hypoglycemia in PwD performing sporting activities a rtCGM should be preferred over isCGM.
Physical activity and exercise are associated with multiple health benefits for people with diabetes [39]. CGM is emerging as an effective tool to preserve glucose homeostasis during and after exercise in PwD, thanks to the opportunity to prevent hypoglycemic events and nocturnal hypoglycemia following physical activity [40, 41]. Both rtCGM and isCGM can be effective tools to help indicate when carbohydrate intake should be started to prevent or treat hypoglycemia during exercise, as emerged in studies involving adolescents and adults with type 1 diabetes [42, 43]. Moreover, the use of rtCGM allows setting alarms to minimize the risk of hypoglycemic episodes at the onset of exercise in type 1 diabetes [40].
Sixth scenario: frail people with diabetes
Figure 6 In frail people with insulin-treated diabetes able to use the devices (either by themselves or with a caregiver) and recurrent hypoglycemic episodes without hypoglycemia awareness, a rtCGM should be suggested in order to reduce the risk of hypoglycemic events. If the awareness of hypoglycemia is preserved, an isCGM may be considered. In the case of suboptimal glycemic control, the choice of a rtCGM or isCGM should be based on the wearability and portability of the devices and individual preferences.
Older adults with diabetes mellitus and long disease duration are at greater risk of prolonged and often unnoticed hypoglycemia, than younger adults. In order to minimize the risk of hypoglycemia, the International Consensus on TIR recommends less stringent glycemic targets in this subgroup [TIR > 50%, TBR (< 70 mg/dl) < 1%, TAR level 1 (> 180 mg/dl) < 50%, TAR level 2 (> 250 mg/dl) < 1] [4]. Evidence from Wireless Innovation in Seniors with Diabetes Mellitus (WISDM) and observational data from DCCT/EDIC study report the effectiveness of CGM in reducing the risk of hypoglycemia up-to one year [15, 18], hyperglycemic excursions and in reaching desired HbA1c levels [44] in older people with type 1 diabetes. The benefits associated with CGM, in terms of improved HbA1c and reduced glycemic variability, are also emerging in studies involving older people with type 2 diabetes using insulin [28, 45]. Particularly, the use of a rtCGM with alerts or predictive alarms by the user and/or the caregiver represents one of the most useful therapeutic tools to avoid hypoglycemia or mitigate its severity in this high-risk population of PwD. Emerging evidence reports the effectiveness of CGM in lowering mean glucose levels during and after hemodialysis in people with type 2 diabetes and stage 3–5 chronic kidney disease (CKD) [46, 47]. This is relevant, as CGM can reveal the daily glucose trends for PwD and CKD on hemodialysis and help them manage daily diet and physical activity.
Discussion
Information collected from CGM is of paramount importance to guide the management of PwD experiencing recurrent hypoglycemia or glucose variability, over the control of HbA1c. CGM allows patients to recognize patterns of blood glucose changes and, consequently, to adjust diet, exercise and insulin dosing accordingly. CGM contributes to improve the lives and health of PwD. On the basis of the growing evidence, its use is currently recognized as the standard of care for people with type 1 diabetes and for a subset of those with insulin-requiring type 2 diabetes [1, 2, 48].
Since the technology of continuous glucose sensors is rapidly evolving, the suggested approach for the responsible use of CGM in people with diabetes is no “one-size-fits-all” [2]. Both rtCGM and isCGM have their own features, abilities, and limitations, that must be considered when selecting the system that meets the personal and clinical needs of people living with diabetes. The present algorithm may help to select the best CGM device based on patients’ clinical characteristics, needs and clinical context, offering a further opportunity of a “tailored” therapy for people with insulin-treated diabetes. The proposed flow-charts have been elaborated on the basis of both current scientific evidence, coming mainly from clinical trials, and principles of good clinical practice, as inspired from daily experience in dealing with PwD.
rtCGM remains the preferred choice over isCGM for monitoring and detection of hypoglycemia, based on the possibility to set predictive alarms/alerts [5]. Interestingly, in the few studies comparing rtCGM with isCGM, an improvement of both TBR and TIR have been associated to rtCGM [49,50,51]. On the other hand, isCGM should be considered as a valuable option over rtCGM at the onset of diabetes, in patients treated with glucose-lowering therapy not associated with hypoglycemic risk or in people with suboptimal glucose control and low risk for hypoglycemia. In these situations, isCGM may provide more data than those obtained by SMBG with an higher ease of use and greater acceptability [5].
Some critical issues still remain and should be taken into account. Most RCTs reported the effects of rtCGM in PwD, whereas RCT data for isCGM are more limited. On the other hand, a higher corpus of evidence from observational/retrospective studies and analyses of registry and population data refer to the use of isCGM in adults with diabetes [34, 52,53,54], whereas real-world evidence for rtCGM use is still restricted to few studies [29, 55].
Future CGM devices may use nanoparticle sensors that demonstrated excellent glucose response in the physiological range and are a promising tool for real-time glucose tracking [56, 57]. In the meantime, the proposed algorithm may represent a good companion for clinicians who desire to optimize the use of CGM in daily clinical practice.
Change history
31 January 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12020-023-03635-w
References
T. Danne, R. Nimri, T. Battelino et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 40, 1631–1640 (2017). https://doi.org/10.2337/dc17-1600
Diabetes Technology, Standards of Medical Care in Diabetes-2023. American Diabetes Association. Diabetes Care 46, S111–S127 (2023)
M.I. Maiorino, S. Signoriello, A. Maio et al. Effects of Continuous Glucose Monitoring on Metrics of Glycemic Control in Diabetes: a Systematic Review with Meta-analysis of Randomized Controlled Trials. Diabetes Care 43, 1146–1156 (2020). https://doi.org/10.2337/dc19-1459
T. Battelino, T. Danne, R.M. Bergenstal et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 42, 1593–1603. https://doi.org/10.2337/dci19-0028
G. Grunberger, J. Sherr, M. Allende et al. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus. Endocr. Pract. 27, 505–537 (2021). https://doi.org/10.1016/j.eprac.2021.04.008
Linea Guida della Associazione dei Medici Diabetologi (AMD), della Società Italiana di Diabetologia (SID) e della Società Italiana di Endocrinologia e Diabetologia Pediatrica (SIEDP). La terapia del diabete mellito di tipo 1. Linea guida pubblicata nel Sistema Nazionale Linee Guida, Roma, 16 marzo 2022
L. Leelarathna, M.L. Evans, S. Neupane et al. Intermittently Scanned Continuous Glucose Monitoring for Type 1 Diabetes. N. Engl. J. Med. 387, 1477–1487 (2022). https://doi.org/10.1056/NEJMoa2205650
L.M. Laffel, L.G. Kanapka, R.W. Beck et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adolescents and Young Adults With Type 1 Diabetes: A Randomized Clinical Trial. JAMA 323, 2388–2396 (2020). https://doi.org/10.1001/jama.2020.6940
T. Martens, R.W. Beck, R. Bailey et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin: A Randomized Clinical Trial. JAMA 325, 2262–2272 (2021). https://doi.org/10.1001/jama.2021.7444
M. Longo, M. Petrizzo, K. Esposito, M.I. Maiorino, Glucose monitoring in diabetes: A suggested algorithm to choice the best treatment option. Diabetes Res. Clin. Pract. 165, 108242 (2020). https://doi.org/10.1016/j.diabres.2020.108242
C.A. van Beers, J.H. DeVries, S.J. Kleijer et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 4, 893–902 (2016). https://doi.org/10.1016/S2213-8587(16)30193-0
R.W. Beck, T. Riddlesworth, K. Ruedy et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA 317, 371–318 (2017). https://doi.org/10.1001/jama.2016.19975
J. Bolinder, R. Antuna, P. Geelhoed-Duijvestijn, J. Kröger, R. Weitgasser, Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 388, 2254–2263 (2016). https://doi.org/10.1016/S0140-6736(16)31535-5
L. Heinemann, G. Freckmann, D. Ehrmann et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 391, 1367–1377 (2017). https://doi.org/10.1016/S0140-6736(18)30297-6
K.M. Miller, L.G. Kanapka, M.R. Rickels et al. Benefit of Continuous Glucose Monitoring in Reducing Hypoglycemia Is Sustained Through 12 Months of Use Among Older Adults with Type 1 Diabetes. Diabetes Technol. Ther. 24, 424–434 (2022). https://doi.org/10.1089/dia.2021.0503
E.H. Serné, I.K. van den Berg, C. Racca et al. Improved Effectiveness of Immediate Continuous Glucose Monitoring in Hypoglycemia-Prone People with Type 1 Diabetes Compared with Hypoglycemia-Focused Psychoeducation Following a Previous Structured Education: A Randomized Controlled Trial. Diabetes Technol. Ther. 25, 50–61 (2022). https://doi.org/10.1089/dia.2022.0232
E. Renard, J.P. Riveline, H. Hanaire, B. Guerci; on behalf of the investigators of France Adoption Clinical Trial, Reduction of clinically important low glucose excursions with a long-term implantable continuous glucose monitoring system in adults with type 1 diabetes prone to hypoglycaemia: the France Adoption Randomized Clinical Trial. Diabetes Obes. Metab. 24, 859–867 (2022). https://doi.org/10.1111/dom.14644
R.E. Pratley, L.G. Kanapka, M.R. Rickels et al. Effect of Continuous Glucose Monitoring on Hypoglycemia in Older Adults With Type 1 Diabetes: A Randomized Clinical Trial. JAMA 323, 2397–2406 (2020). https://doi.org/10.1001/jama.2020.6928
E. Bosi, P. Choudhary, H.W. de Valk et al. Efficacy and safety of suspend-before-low insulin pump technology in hypoglycaemia-prone adults with type 1 diabetes (SMILE): an open-label randomised controlled trial. Lancet Diabetes Endocrinol. 7, 462–472 (2019). https://doi.org/10.1016/S2213-8587(19)30150-0
S.A. Little, J. Speight, L. Leelarathna et al. Sustained Reduction in Severe Hypoglycemia in Adults With Type 1 Diabetes Complicated by Impaired Awareness of Hypoglycemia: Two-Year Follow-up in the HypoCOMPaSS Randomized Clinical Trial. Diabetes Care 41, 1600–1607 (2018). https://doi.org/10.2337/dc17-2682
M.J. Davies, V.R. Aroda, B.S. Collins et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 45, 2753–2786 (2022). https://doi.org/10.2337/dci22-0034
R.I.G. Holt, J.H. DeVries, A. Hess-Fischl et al. The Management of Type 1 Diabetes in Adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 44, 2589–2625 (2021). https://doi.org/10.2337/dci21-0043
R. Franceschi, V. Cauvin, L. Stefani, F. Berchielli, M. Soffiati, E. Maines, Early Initiation of Intermittently Scanned Continuous Glucose Monitoring in a Pediatric Population With Type 1 Diabetes: A Real World Study. Front Endocrinol. (Lausanne) 17(13), 907517 (2022). https://doi.org/10.3389/fendo.2022.907517
A. Champakanath, H.K. Akturk, G.T. Alonso, J.K. Snell-Bergeon, V.N. Shah, Continuous Glucose Monitoring Initiation Within First Year of Type 1 Diabetes Diagnosis Is Associated With Improved Glycemic Outcomes: 7-Year Follow-Up Study. Diabetes Care 45, 750–753 (2022). https://doi.org/10.2337/dc21-2004
G. Mulinacci, G.T. Alonso, J.K. Snell-Bergeon, V.N. Shah, Glycemic Outcomes with Early Initiation of Continuous Glucose Monitoring System in Recently Diagnosed Patients with Type 1 Diabetes. Diabetes Technol. Ther. 21, 6–10 (2019). https://doi.org/10.1089/dia.2018.0257
S.R. Patton, A.E. Noser, E.M. Youngkin, S. Majidi, M.A. Clements, Early Initiation of Diabetes Devices Relates to Improved Glycemic Control in Children with Recent-Onset Type 1 Diabetes Mellitus. Diabetes Technol. Ther. 21, 379–384 (2019). https://doi.org/10.1089/dia.2019.0026
R.W. Beck, T.D. Riddlesworth, K. Ruedy et al. Continuous Glucose Monitoring Versus Usual Care in Patients With Type 2 Diabetes Receiving Multiple Daily Insulin Injections: A Randomized Trial. Ann. Intern. Med. 167, 365–374 (2017). https://doi.org/10.7326/M16-2855
R.M. Bergenstal, D.M. Mullen, E. Strock et al. Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J. Diabetes Complications. 36, 108106 (2022). https://doi.org/10.1016/j.jdiacomp.2021.108106
A.J. Karter, M.M. Parker, H.H. Moffet, L.K. Gilliam, R. Dlott, Association of Real-time Continuous Glucose Monitoring With Glycemic Control and Acute Metabolic Events Among Patients With Insulin-Treated Diabetes. JAMA 325, 2273–2284 (2021). https://doi.org/10.1001/jama.2021.6530
A.L. McCall, D.C. Lieb, R. Gianchandani et al. Management of Individuals With Diabetes at High Risk for Hypoglycemia: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 15, 529–562 (2023). https://doi.org/10.1210/clinem/dgac596
S.J. Moon, K.S. Kim, W.J. Lee, M.Y. Lee, R. Vigersky, C.Y. Park, Efficacy of intermittent short-term use of a real-time continuous glucose monitoring system in non-insulin-treated patients with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 25, 110–120 (2023). https://doi.org/10.1111/dom.14852
R.A. Vigersky, S.J. Fonda, M. Chellappa, M.S. Walker, N.M. Ehrhardt, Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 35, 32–38 (2012). https://doi.org/10.2337/dc11-1438
Y. Gao, M. Zhou, X. Xu, W.Y. Chen, Effects of flash glucose monitoring on glycemic control in participants with diabetes mellitus: A meta-analysis of randomized controlled trials. J. Diabetes Complications. 36, 108314 (2022). https://doi.org/10.1016/j.jdiacomp.2022
E. Bosi, G. Gregori, C. Cruciani, C. Irace, P. Pozzilli, R. Buzzetti, The use of flash glucose monitoring significantly improves glycemic control in type 2 diabetes managed with basal bolus insulin therapy compared to self-monitoring of blood glucose: A prospective observational cohort study. Diabetes Res. Clin. Pract. 183, 109172 (2022). https://doi.org/10.1016/j.diabres.2022.109885
R. Aronson, R.E. Brown, L. Chu et al. IMpact of flash glucose Monitoring in people with type 2 Diabetes Inadequately controlled with non-insulin Antihyperglycaemic ThErapy (IMMEDIATE): A randomized controlled trial. Diabetes Obes. Metab. 25, 1024–1031 (2023). https://doi.org/10.1111/dom.14949
T. Haak, H. Hanaire, R. Ajjan, N. Hermanns, J.P. Riveline, G. Rayman, Flash Glucose-Sensing Technology as a Replacement for Blood Glucose Monitoring for the Management of Insulin-Treated Type 2 Diabetes: a Multicenter, Open-Label Randomized Controlled Trial. Diabetes Ther. 8, 55–73 (2017). https://doi.org/10.1007/s13300-016-0223-6
D.S. Feig, L.E. Donovan, R. Corcoy et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 390, 2347–2359 (2017). https://doi.org/10.1016/S0140-6736(17)32400-5
R.M. García-Moreno, P. Benítez-Valderrama, B. Barquiel et al. Efficacy of continuous glucose monitoring on maternal and neonatal outcomes in gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials. Diabet. Med. 39, e14703 (2022). https://doi.org/10.1111/dme.14703
S.R. Colberg, R.J. Sigal, J.E. Yardley et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 39, 2065–2079 (2016). https://doi.org/10.2337/dc16-1728
O. Schubert-Olesen, J. Kröger, T. Siegmund, U. Thurm, M. Halle, Continuous Glucose Monitoring and Physical Activity. Int. J. Environ. Res. Public Health 19, 12296 (2022). https://doi.org/10.3390/ijerph191912296
O. Moser, M.C. Riddell, M.L. Eckstein et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 63, 2501–2520 (2020). https://doi.org/10.1007/s00125-020-05263-9
M.A. Burckhardt, T. Chetty, G.J. Smith et al. Use of Continuous Glucose Monitoring Trends to Facilitate Exercise in Children with Type 1 Diabetes. Diabetes Technol. Ther. 21, 51–55 (2019). https://doi.org/10.1089/dia.2018.0292
M.C. Riddell, J. Milliken, Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol. Ther. 13, 819–825 (2011). https://doi.org/10.1089/dia.2011.0052
R.A. Gubitosi-Klug, B.H. Braffett, I. Bebu et al. Continuous glucose monitoring in adults with type 1 diabetes with 35 years duration from the DCCT/EDIC Study. Diabetes Care 45, 659–665 (2022). https://doi.org/10.2337/dc21-0629
K.J. Ruedy, C.G. Parkin, T.D. Riddlesworth, C. Graham; DIAMOND Study Group, Continuous Glucose Monitoring in Older Adults With Type 1 and Type 2 Diabetes Using Multiple Daily Injections of Insulin: Results From the DIAMOND Trial. J. Diabetes Sci. Technol. 11, 1138–1146 (2017). https://doi.org/10.1177/1932296817704445
E. Sobngwi, G. Ashuntantang, E. Ndounia et al. Continuous interstitial glucose monitoring in nondiabetic subjects with end-stage renal disease undergoing maintenance haemodialysis. Diabetes Res. Clin. Pract. 90, 22–25 (2010). https://doi.org/10.1016/j.diabres.2010.06.001
M. Joubert, C. Fourmy, P. Henri, M. Ficheux, T. Lobbedez, Y. Reznik, Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res. Clin. Pract. 107, 348–354 (2015). https://doi.org/10.1016/j.diabres.2015.01.026
M. La Noce, G.F. Nicoletti, G. Papaccio, V. Del Vecchio, F. Papaccio, Insulitis in Human Type 1 Diabetic Pancreas: From Stem Cell Grafting to Islet Organoids for a Successful Cell-Based Therapy. Cells 11, 3941 (2022). https://doi.org/10.3390/cells11233941
M.M. Visser, S. Charleer, S. Fieuws et al. Effect of switching from intermittently scanned to real-time continuous glucose monitoring in adults with type 1 diabetes: 24-month results from the randomised ALERTT1 trial. Lancet Diabetes Endocrinol. 11, 96–108 (2023). https://doi.org/10.1016/S2213-8587(22)00352-7
A. Hásková, L. Radovnická, L. Petruželková et al. Real-time CGM Is Superior to Flash Glucose Monitoring for Glucose Control in Type 1 Diabetes: The CORRIDA Randomized Controlled Trial. Diabetes Care 43, 2744–2750 (2020). https://doi.org/10.2337/dc20-0112
M. Reddy, N. Jugnee, A. El Laboudi, E. Spanudakis, S. Anantharaja, N. Oliver, A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with type 1 diabetes and impaired awareness of hypoglycaemia. Diabet. Med. 35, 483–490 (2018). https://doi.org/10.1111/dme.13561
H. Deshmukh, E.G. Wilmot, R. Gregory et al. Effect of flash glucose monitoring on glycemic control, hypoglycemia, diabetes-related distress, and resource utilization in the Association of British Clinical Diabetologists (ABCD) nationwide audit. Diabetes Care 43, 2153–2160 (2020). https://doi.org/10.2337/dc20-0738
R. Roussel, J.P. Riveline, E. Vicaut et al. Important drop in rate of acute diabetes complications in people with type 1 or type 2 diabetes after initiation of flash glucose monitoring in France: the RELIEF study. Diabetes Care 44, 1368–1376 (2021)
S. Charleer, C. De Block, L. Van Huffel et al. Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care 43, 389–397 (2020). https://doi.org/10.2337/dc19-1610
D. Sandig, J. Grimsmann, C. Reinauer et al. Continuous glucose monitoring in adults with type 1 diabetes: real-world data from the German/Austrian Prospective Diabetes Follow-Up Registry. Diabetes Technol. Ther. 22, 602–612 (2020)
https://www.fda.gov/medical-devices/510k-clearances/june-2020-510k-clearances (accessed on May the 1st 2023)
L.V. Le, G.S. Chendke, S. Gamsey, N. Wisniewski, T.A. Desai, Near-Infrared Optical Nanosensors for Continuous Detection of Glucose. J. Diabetes Sci. Technol. 14, 204–211 (2020). https://doi.org/10.1177/1932296819886928
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Consortia
Contributions
M.I.M. and K.E. conceived the algorithm and wrote the manuscript. All authors contributed to drafting the manuscript, revised it for intellectual content and approved its final version.
Corresponding author
Ethics declarations
Conflict of interest
M.I.M. received speaker fees from Novo Nordisk and Ely Lilly. R.B. received fees from Medtronic, Abbott, Novo Noridsk, Ely Lilly, Astrazeneca and Boehringer Ingelheim Pharmaceuticals. C.I. has provided advisory board services for Novo Nordisk, Lilly, Abbott, Menarini, Ascensia, and Senseonics, and has received speaker fees for Novo Nordisk, Abbott, Ascensia, Lilly, and Boehringer Ingelheim Pharmaceuticals. L.L. has received speaker fees from Abbott, Astra Zeneca, Eli Lilly, Medtronic, Menarini, Novo Nordisk, Roche Diabetes Care, Sanofi Aventis and Terumo and has provided advisory services to Abbott, Eli Lilly, Novo Nordisk, Roche Diabetes Care, and Sanofi Aventis. N.N. received fees from Eli Lilly and Novo Nordisk. K.E. received speaker fees from Novo Nordisk and Ely Lilly and has provided consultancy services to Roche and Medtronic. D.P. has nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A full list of members and their affiliations appears in the Supplementary Information.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maiorino, M.I., Buzzetti, R., Irace, C. et al. An updated algorithm for an effective choice of continuous glucose monitoring for people with insulin-treated diabetes. Endocrine 82, 215–225 (2023). https://doi.org/10.1007/s12020-023-03473-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03473-w