Abstract
Purpose
Tolvaptan, a selective vasopressin V2-receptor antagonist, is approved for the treatment of SIADH-related hyponatremia, but its use is limited. The starting dose is usually 15 mg/day, but recent clinical experience suggests a lower starting dose (<15 mg/day) to reduce the risk of sodium overcorrection. However, long-term low-dose efficacy and safety has not been explored, so far. Aim of our study is to characterize safety and efficacy of long-term SIADH treatment with low-dose Tolvaptan.
Methods
We retrospectively evaluated 11 patients receiving low-dose Tolvaptan (<15 mg/day) for chronic SIADH due to neurological, idiopathic and neoplastic causes. Plasma sodium levels were measured before and 1, 3, 5, 15 and 30 days after starting Tolvaptan and then at 3-month intervals. Anamnestic and clinical data were collected.
Results
Mean time spanned 27.3 ± 29.8 months (range 6 months-7 years). Mean plasma sodium levels were within normal range 1, 3 and 6 months after starting Tolvaptan as well as after 1, 2, 3, 5 and 7 years of therapy. Neither osmotic demyelination syndrome nor overcorrection were observed. Plasma sodium levels normalization was associated with beneficial clinical effects. Neurological patients obtained seizures disappearance, improvement in neurological picture and good recovery from rehabilitation. Neoplastic patients were able to start chemotherapy and improved their general condition. Patients did not show hypernatremia during long-term follow-up and reported mild thirst and pollakiuria.
Conclusions
The present study shows that long-term low-dose Tolvaptan is safe and effective in SIADH treatment. No cases of overcorrection were documented and mild side effects were reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyponatremia is the commonest electrolyte abnormality in hospitalized and community patients and it has been associated with high morbidity and mortality [1, 2]. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is a major cause of hyponatremia, secondary to a variety of neurological, pulmonary, infective, and neoplastic disorders, as well as drug administration. SIADH may be associated with symptoms such as nausea, vomiting, unstable gait, vertigo, disorientation, and impairment of cognitive functions but it can also present with severe neurological signs, such as seizures and loss of consciousness [1,2,3].
Conventional SIADH treatment associated with mild to moderate hyponatremia consists of fluid restriction and rarely demeclocycline or urea, whereas hypertonic saline is employed in cases of severe and symptomatic hyponatremia [1, 2]. Tolvaptan is a selective antagonist of the vasopressin V2-receptor that blocks vasopressin activity on renal collecting tubules resulting in an increase in plasma sodium concentrations [1, 2]. The drug is approved for the treatment of adult patients with SIADH, but its use is limited. Tolvaptan starting dose is usually 15 mg/day, but recent clinical experience evidenced that lower doses may be sufficient to normalize sodium plasma levels and suggested a starting dose of 7.5 mg/day [4,5,6]. At present, only few reports in literature describe chronic SIADH long-term treatment with low-dose Tolvaptan [7,8,9]. We therefore aimed at better characterizing safety and efficacy of long-term low-dose (<15 mg/die) Tolvaptan treatment in 11 patients with chronic hyponatremia SIADH-related and reviewed related literature.
Material and methods
We retrospectively evaluated patients who received chronically low-dose Tolvaptan (Samsca, Zhejiang Otsuka Pharmaceutical, Tokyo, Japan) therapy for chronic hyponatremia secondary to SIADH (Tab. 1). The inclusion criteria were:
-
SIADH diagnosis with plasma sodium levels <135 mmol/L for ≥30 days;
-
treatment with Tolvaptan for >3 months at daily doses <15 mg/day (defined as low-dose Tolvaptan).
Exclusion criteria were: age <18 years old; medication interval >1 week; severe liver or kidney dysfunction before treatment.
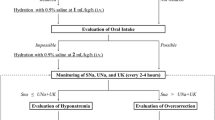
SIADH was diagnosed according to the guideline criteria [10]. All patients had been unsuccessfully previously treated with conventional treatments: i.v. and/or enteral sodium supplementation and water restriction (<1.5 l/d) before Tolvaptan administration. Fluid intake was not limited and no extra oral sodium supplements were indicated. In patients receiving enteral nutrition, water daily balance was carefully monitored. All patients routinely took other types of drugs including those favouring hyponatremia (i.e., anti-epileptics). Because some SIADH causes may be transient in nature (i.e., subarachnoid hemorrhage) all patients received dosage adjustment according to plasma sodium levels in the short and long-term to see if the hyponatremia recurred as evidence of chronic SIADH.
Plasma sodium levels were measured before starting Tolvaptan and then at 1, 3, 5, 15 and 30 days. Natremia was then measured at 3-month intervals. Anamnestic and clinical data were collected before and during treatment: age, sex, SIADH cause, blood pressure, heart rate, body weight and symptoms due to hyponatremia. In addition, the following laboratory data were collected: glucose, potassium, creatinine, serum urea nitrogen, uric acid, alanine aminotransferase.
In patients evaluated during traumatic brain injury (TBI) post-acute phase, clinical picture during Tolvaptan treatment was measured by the following scales: Functional Independence Measure (FIM) scoring from 18 (complete dependence) to 126 (complete independence); Disability Rating Scale (DRS) scoring from 0 (no disability) to 9 (extreme vegetative state); Levels of Cognitive Functioning (LCF) with 8 cognitive levels assessing the patient’s cognitive function (8 = purposeful, appropriate response) [11]. Modifications in performance status of oncologic patients during Tolvaptan therapy was assessed by the ECOG Scale (Eastern Cooperative Oncology Group) [12]. Sodium overcorrection was defined by a Na+ increase >10 mmol/L during the first 24 h and >8 mmol/L during every 24 h thereafter [8]. Hyponatremia was divided into: mild (Na+ between 130 and 135 mmol/L), moderate (Na+ between 125 and 129 mmol/L) and severe (Na+ <125 mmol/L) [10].
Statistical analysis was performed by means of the Fisher’s exact test for qualitative measures, by T test for parametric distribution of quantitative measures and by Kruskal–Wallis test for non-parametric distribution of quantitative measures. P values <0.05 were considered to indicate statistical significance. The study was approved by the local ethical committee and informed consent was obtained from the subjects or next of kin.
The drug was administered orally in 7 cases and enterally by percutaneous endoscopic gastrostomy (PEG) in 4 cases.
Results
We evaluated 11 patients (4 F, 7 M, mean age = 55.72 ± 22.8 years). SIADH was due to neurological causes in eight individuals (4 severe TBI; 1 subarachnoid hemorrhage; 1 cerebral hemorrhage; 1 stereotactic radiosurgery for glioma recurrence; 1 multi-infarct encephalopathy), to neoplastic causes in 2 (small cell lung cancer, SCLC), and to idiopathic cause in one patient (Table 1). Patients started oral Tolvaptan at 15 mg/d in three cases (2 post-acute TBI patients and 1 neoplastic subject), 7.5 mg/d in seven cases, and 3.75 mg/d in one neurological case.
Six out of eight neurological SIADH patients had seizures treated with antiepileptic drugs. In these patients, hyponatremia was associated with seizure crisis (6 cases), cognitive impairment and poor motor initiative (7 cases), drowsiness (7 cases) and reduced response to the rehabilitation (3 cases).
In neoplastic patients, hyponatremia delayed the start of chemotherapy treatment, that was possible after Tolvaptan administration; in one neoplastic patient hyponatremia caused a single seizure episode with fall and left posterior glenohumeral dislocation. In idiopathic SIADH, hyponatremia caused losses of consciousness and repeated falls with bone fractures requiring some hospitalizations.
Mean plasma sodium levels before starting Tolvaptan were 127.9 ± 3.2 mmol/L (range: 124–132 mmol/L). In particular, three patients had severe, 4 moderate and 4 mild hyponatremia (130 mmol/L ≤ Na+ <132 mmol/L) (Table 1). Basal sodium plasma levels were slightly, but not significantly, lower in the three patients starting with Tolvaptan 15 mg/d (mean = 124.7 ± 1.15 mmol/L) compared to the eight patients starting with Tolvaptan 7.5 or 3.75 mg/d (mean = 129.1 ± 2.9 mmol/L). Mean plasma sodium levels significantly increased to 132.6 ± 3.7 mmol/L (p < 0.01 vs. basal levels) (range: 128–139 mmol/L) and to 135.1 ± 2.7 mmol/L (p < 0.01 vs. basal levels) (range: 131–139 mmol/L) after 1 and 3 days of Tolvaptan treatment, respectively.
The sodium correction rate was slightly but not significantly higher in the 15 mg/d as compared to 7.5 mg/d group after 1 day group (mean: 6.7 ± 1.2 mmol/L and 4.0 ± 2.0 mmol/L, respectively). Similar results were found after 3 days (mean: 9.7 ± 4.0 mmol/L and 6.3 ± 1.8 mmol/L).
The sodium correction rate after 1 day in patients with severe hyponatremia was slightly but not significantly higher as compared to patients with mild-moderate hyponatremia (mean: 5.3 ± 1.2 mmol/L and 4.9 ± 2.5 mmol/L, respectively). Similar results were found after 3 days (mean: 11 ± 1.7 mmol/L and 5.8 ± 1.5 mmol/L, respectively). All three patients starting with 15 mg/d dose had sodium plasma levels ≥130 mmol/L after 1 day of treatment vs. 6 out 8 individuals on 7.5/3.75 mg/d dose (however 4 out 8 patients had Na+ basal levels between 130 and 132 mmol/l and the difference was not statistically significant). After 3 days of treatment, all individuals presented with plasma sodium levels ≥130 mmol/L (Table 2).
Follow-up
We followed all patients up for at least 6 months (from 6 to 84 months) with a mean treatment time of 27.3 ± 29.8 months and sodium plasma levels maintained within a low-normal range (Figs. 1 and 2). Median plasma sodium levels at each point are described in Table 2, as well percentage of patients reaching normonatraemia during follow-up. Two patients managed to stop Tolvaptan and plasma sodium levels remained within reference range after drug withdrawal. In particular, one TBI patient stopped therapy after 6 months thanks to an optimal response recovery after rehabilitation and improvement of neurological conditions, whereas one neoplastic patient stopped Tolvaptan after 6 months thanks to response to chemotherapy. In patients receiving longer treatment, sodium plasma levels were stable up to 7 years of therapy, with few dose adjustments (Figs. 2 and 3), frequently concomitant with modifications in anti-epileptic drug treatment (Table 2).
Mean sodium plasma levels under Tolvaptan treatment for more than 30 days. Black dots: mean sodium plasma levels of 11 patients. Gray squares: mean sodium plasma levels of five patients. White dots: mean sodium plasma levels of four patients. Black triangles: sodium plasma levels of one patient. Data are displayed as mean sodium plasma levels ± standard error of the mean
Mean Tolvaptan dose (mg/d) during follow-up. Data are displayed as mean Tolvaptan dose ± standard error of the mean. Black bars: mean Tolvaptan dose of 11 patients. White bars: mean Tolvaptan dose of five patients. Gray bars: mean Tolvaptan dose of four patient. Striped bars: mean Tolvaptan dose of one patient
Clinical benefits
In all patients, normalization of sodium plasma levels was associated with beneficial clinical effects (Table 3). The eight neurological patients obtained seizures disappearance and improvement in neurological picture. Patients and their families reported an improvement in attention, memory, motor skills and quality of life. Two patients treated in the TBI post-acute phase showed little improvement in cognitive and motor functioning scales, with slight but not significant changes in the evaluated scales (at rehabilitation admission FIM = 18/126, LCF = 3/8, DRS = 22/29; at rehabilitation discharge FIM = 30–35/126, LCF = 3–4/8, DRS = 20–16/29). One post-TBI patient had a good response to rehabilitation, presenting with extremely severe disability at admission (FIM = 18/126; LCF = 3/8, DRS = 21/29) and moderate disability at discharge (FIM = 101/126; LCF = 8/8, DRS = 4/29).
After the start of Tolvaptan treatment, neoplastic patients were able to start chemotherapy and their general condition improved. ECOG improved from 2 to 0 points in one neoplastic patient thanks to chemotherapy and hyponatremia normalization and she obtained seizures disappareance. In this patient Tolvaptan was subsequently discontinued. The other neoplastic patient had a stable ECOG (1 point) but developed neutropenia during chemotherapy that delayed subsequent cycles (Table 3).
The patient with idiopathic SIADH reported improvement in general well-being and physical performance; the losses of consciousness and the repeated falls did not occur any more (Table 3).
Adverse events
Osmotic demyelination syndrome and overcorrection were not observed during the first 24–72 h nor during long-term follow-up. No changes in body weight, heart rate, blood pressure and biochemical parameters were observed during follow-up. One patient reported thirst and pollakiuria for 1 years. No liver toxicity occurred. After 18 months from the start of Tolvaptant treatment, epileptic crisis developed in one male neurological patient displaying concomitant hyponatremia (132 mmol/L) because of Tolvaptan dose reduction made by himself (from 7.5 mg/d for 4 day a week to 7.5 mg/d for 2 days a week). Sodium plasma levels returned within normal range after the subsequent increase of Tolvaptan dose and seizures disappeared. The same episode occurred in one female neurological patient after 60 months of treatment (Na+ 133 mmol/L after Tolvaptan dose reduction made by herself). Seizure disappeared and Na+ normalized after increase in Tolvaptan dose.
Discussion
The present study demonstrates safety and efficacy of short- and long-term treatment (up to 84 months) with low-dose Tolvaptan (3.75–15 mg/d) in patients with chronic SIADH due to neurological, idiopathic and neoplastic causes, not responsive to conventional treatment. Indeed, in our study all patients achieved normal sodium plasma levels despite Tolvaptan was started at doses lower that those recommended by current guidelines. In addition, few adverse events were recorded. As a first-line treatment, the traditional option is fluid restriction. However, often due to specific patient characteristics, this strategy is not possible to apply, or on the other hand, it is not very effective, sometimes because of the low therapeutic adherence. In consequence, we must resource to alternative pharmacological therapies, such as vaptans or urea. In clinical practice urea is not widely extended maybe because of the lack of convincing evidences with limited and heterogeneous studies [13]. Therefore we chose to use tolvaptan, also driven by the fact that urea is not palatable with poor adherence to therapy.
Nowadays, only few data on long-term Tolvaptan administration in SIADH have been reported (Table 4). In addition, only one report described the efficacy of low-dose Tolvaptan in 4 SIADH patients with age >90 years old with a follow-up of 2 years [7,8,9] whereas the other papers report data on chronic treatment with standard dose of Tolvaptan. To our knowledge, our study reports the longest follow up with low-dose Tolvaptan (up to 84 months) and, differently to other works, in the present study all patients used Tolvaptan dose <15 mg/d at the last follow-up.
Usually, Tolvaptan 15 mg/d represents the starting dose that can be increased to 30–60 mg/d according to sodium plasma levels. In our study, sodium plasma levels normalized both with starting Tolvaptan doses of 15 mg/d (three patients) and with lower doses (7.5 mg and 3.75 mg/d). In addition, doses >15 mg/d were not necessary. These data suggest that in SIADH patients a Tolvaptan dose of 7.5 mg/d might be effective in adequately control natremia in the short-term. Our results are in line with those reported by a study evaluating 23 SCLC patients with hyponatremia due to SIADH. In these settings, sodium plasma levels were similar in patients receiving 3.75 mg/d and in those receiving >3.75 mg/d Tolvaptan after 3 days of treatment [14].
Our data suggest that Tolvaptan can be co-administered, even in the long- term, with drugs determining hyponatremia without further adverse events. In particular, in epileptic subjects, keeping normal sodium plasma levels improved clinical status and led to seizures disappearance. These aspects may reduce medical costs due to hospitalization and improve medical condition [13,14,15,16,17,18,19,20]. In neurological patients, natremia normalization significantly improved neurological symptoms and quality of life of the patients and their caregivers. Verbalis et al. indicated that SIADH patients treated with Tolvaptan 15 mg/d (or higher doses) had a statistically significant clinical improvement in the Physical Component Summary (PCS) after 30 days of therapy in comparison with placebo. They also showed that Mental Component Summary (MCS) improvement was higher in Tolvaptan group in comparison with placebo, and the difference was near to statistical significance [21].
Our study supports the hypothesis that Tolvaptan may positively impact the outcome of hyponatraemic neoplastic patients, allowing to start chemotherapy earlier as previously described [22]. In addition, patients treated with chemotherapic drugs often need adequate hydration, contraindicating fluid restriction. In our study, natremia was well controlled by low Tolvaptan dose even in cancer patients. In addition, chemotherapy may induce SIADH (i.e., cisplatin) [23]. Our study also suggests that Tolvaptan reduction should be attempted as soon as a tumor response to chemotherapy is recorded. De Las Peñas et al. suggest that in SCLC patients Tolvaptan dose should be reduced from 15 mg/d to 7.5 mg/d if hyponatremia improves after 7 days of treatment, and then stopped in case of further improvement after 14 days [13]. This approach may reduce the risk of overcorrection and medical costs, improving symptoms, morbidity and mortality at the same time [14,15,16,17,18,19,20, 23]. Our data also suggest that Tolvaptan dose reduction is possible when hyponatremia causes can be successfully managed, allowing an improvement in patient clinical picture.
The present data indicate that long-term low-dose Tolvaptan (<15 mg/d) is safe, both in the short- and in the long-term. Indeed, in our series overcorrection and osmotic demyelination syndrome did not occur. Indeed, when the plasma sodium concentration increases too rapidly, osmotic demyelination syndrome may develop and permanent brain damage may occur [14]. However, overcorrection may also depend on the severity of basal hyponatremia [1, 2, 13, 14] and none of our patients had sodium plasma levels <120 mmol/L at study entry. In addition, in keeping with literature data, sodium correction rate was slightly lower in patients receiving low-dose Tolvaptan (3.75–7.5 mg/d) as compared to patients receiving Tolvaptan 15 mg/d [4,5,6, 24]. On the opposite, Tzoulis et al. showed similar mean daily correction rates in patients with both 15 mg/d and 7.5 mg/d. This finding may be due to patient selection, since Tolvaptan 7.5 mg/d was delivered to patients with severe hyponatremia [25].
Low Tolvaptan dose was safe also in severe neurological patients in whom the probability of overcorrection is high, due to the lack of thirst. Therefore, such patients require a strict clinical and biochemical monitoring especially in the first phase of treatment. In these patients, PEG was a useful tool for Tolvaptan administration, helping drug management and fluid administration.
Tolvaptan was safe in all treated patients, regardless of age and drug co-administration, and it did not cause any significant change in other biochemical parameters or blood pressure, as previously reported [7, 26]. In particular, long term combination with antiepileptic, anti-hypertensive, and/or antiplatelet/anticoagulant drugs did not change the efficacy neither increased the incidence of adverse effects of these drugs. Indeed, we did not observe serious adverse events in neither the short- nor the long-term period. The most common adverse events were thirst and pollakiuria, both of which disappeared, as previously reported [9, 27]; no liver toxicity occurred.
It is well known that chronic hyponatremia is associated with significant morbidity including increased prolonged length of stay, and increased readmissions to hospital, as well as cognitive dysfunction, gait instability, and fractures [28]. The clinical benefit demonstrated in our patients suggest that treatment of chronic hyponatremia with low dose of Tovalptan may safe and efficacy in preventing morbidity due this conditions. Limits of the study are the small sample size and the retrospective design. Prospective studies with larger numbers of patients are needed to confirm these data
Conclusions
The present study demonstrates safety and efficacy of short- and long-term treatment (up to 84 months) with low-dose tolvaptan (<15 mg/d) in patients with SIADH due to neurological, idiopathic and neoplastic causes. No cases of overcorrection were found and the most common adverse events were thirst and pollakiuria. Low-dose Tolvaptan can be co-administered with drugs determining hyponatremia without adverse events in both short- and long-term period. PEG was a useful tool for the administration of Tolvaptan in severe neurological subjects.
Data availability
Data that support the findings of this study are available on request from the corresponding author.
References
R.W. Schrier, P. Gross, M. Gheorghiade, T. Berl, J.G. Verbalis, F.S. Czerwiec, C. Orlandi; SALT Investigators, Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N. Engl. J. Med. 355(20), 2099–2112 (2006). https://doi.org/10.1056/NEJMoa065181
P. Gross, Clinical management of SIADH. Ther. Adv. Endocrinol. Metab. 3(2), 61–73 (2012). https://doi.org/10.1177/2042018812437561
P. Grant, J. Ayuk, P.M. Bouloux, M. Cohen, I. Cranston, R.D. Murray, A. Rees, N. Thatcher, A. Grossman, The diagnosis and management of inpatient hyponatraemia and SIADH. Eur. J Clin. Investig. 45(8), 888–894 (2015). https://doi.org/10.1111/eci.12465
V. Chatzimavridou-Grigoriadou, S. Al-Othman, G. Brabant, A. Kyriacou, J. King, F. Blackhall, P.J. Trainer, C.E. Higham, Clinical experience of the efficacy and safety of low-dose tolvaptan therapy in a UK tertiary oncology setting. J. Clin. Endocrinol. Metab. 106(11), e4766–e4775 (2021). https://doi.org/10.1210/clinem/dgab131
R.M. Hanna, J.C. Velez, A. Rastogi, M.K. Nguyen, M.K. Kamgar, K. Moe, F. Arman, H. Hasnain, N. Nobakht, U. Selamet, I. Kurtz, Equivalent efficacy and decreased rate of overcorrection in patients with syndrome of inappropriate secretion of antidiuretic hormone given very low-dose tolvaptan. Kidney Med. 2(1), 20–28 (2019). https://doi.org/10.1016/j.xkme.2019.09.004
B. Harbeck, U. Lindner, C.S. Haas, Low-dose tolvaptan for the treatment of hyponatremia in the syndrome of inappropriate ADH secretion (SIADH). Endocrine 53(3), 872–873 (2016). https://doi.org/10.1007/s12020-016-0912-y
Y.H. Liu, X.B. Han, Y.H. Fei, H.T. Xu, Long-term low-dose tolvaptan treatment in hospitalized male patients aged >90 years with hyponatremia: report on safety and effectiveness. Medicine 96(52), e9539 (2017). https://doi.org/10.1097/MD.0000000000009539
S. Büttner, J. Bachmann, H. Geiger, N. Obermüller, Long-term vaptan treatment of idiopathic SIADH in an octogenarian. J. Clin. Med. 6(3), 28 (2017). https://doi.org/10.3390/jcm6030028
T. Berl, F. Quittnat-Pelletier, J.G. Verbalis, R.W. Schrier, D.G. Bichet, J. Ouyang, F.S. Czerwiec; SALTWATER Investigators, Oral tolvaptan is safe and effective in chronic hyponatremia. J. Am. Soc. Nephrol. 21(4), 705–712 (2010). https://doi.org/10.1681/ASN.2009080857
G. Spasovski, R. Vanholder, B. Allolio, D. Annane, S. Ball, D. Bichet, G. Decaux, W. Fenske, E.J. Hoorn, C. Ichai, M. Joannidis, A. Soupart, R. Zietse, M. Haller, S. van der Veer, W. Van Biesen, E. Nagler; Hyponatraemia Guideline Development Group, Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur. J. Endocrinol. 170(3), G1–G47 (2014). https://doi.org/10.1530/EJE-13-1020
M. Bondanelli, M.R. Ambrosio, L. Cavazzini, A. Bertocchi, M.C. Zatelli, A. Carli, D. Valle, N. Basaglia, E.C. Uberti, Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J. Neurotrauma 24(11), 1687–1697 (2007). https://doi.org/10.1089/neu.2007.0343
M.M. Oken, R.H. Creech, D.C. Tormey, J. Horton, T.E. Davis, E.T. McFadden, P.P. Carbone, Toxicity and response criteria of the eastern cooperative oncology group. Am. J. Clin. Oncol. 5(6), 649–655 (1982)
E.J. Hoorn, R. Zietse, Diagnosis and treatment of hyponatremia: compilation of the guidelines. J. Am. Soc. Nephrol. 28(5), 1340–1349 (2017). https://doi.org/10.1681/ASN.2016101139
R. De Las Peñas, S. Ponce, F. Henao, C. Camps Herrero, E. Carcereny, Y. Escobar Álvarez, C.A. Rodríguez, J.A. Virizuela, R. López López, SIADH-related hyponatremia in hospital day care units: clinical experience and management with tolvaptan. Supportive Care Cancer 24(1), 499–507 (2016). https://doi.org/10.1007/s00520-015-2948-6
N. Janicic, J.G. Verbalis, Evaluation and management of hypo-osmolality in hospitalized patients. Endocrinol. Metab. Clin. N. Am. 32(2), 459–vii (2003). https://doi.org/10.1016/s0889-8529(03)00004-5
L. Klein, C.M. O’Connor, J.D. Leimberger, W. Gattis-Stough, I.L. Piña, G.M. Felker, K.F. Adams Jr, R.M. Califf, M. Gheorghiade; OPTIME-CHF Investigators, Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation 111(19), 2454–2460 (2005). https://doi.org/10.1161/01.CIR.0000165065.82609.3D
B. Renneboog, W. Musch, X. Vandemergel, M.U. Manto, G. Decaux, Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am. J. Med. 119(1), 71.e1–71.e718 (2006). https://doi.org/10.1016/j.amjmed.2005.09.026
S.M. Doshi, P. Shah, X. Lei, A. Lahoti, A.K. Salahudeen, Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am. J. Kidney Dis. 59(2), 222–228 (2012). https://doi.org/10.1053/j.ajkd.2011.08.029
J.G. Verbalis, S.R. Goldsmith, A. Greenberg, C. Korzelius, R.W. Schrier, R.H. Sterns, C.J. Thompson, Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am. J. Med. 126(10 Suppl 1), S1–S42 (2013). https://doi.org/10.1016/j.amjmed.2013.07.006
P. Ren, Q. Yang, The role of tolvaptan in managing hyponatremia in small cell lung cancer patients with SIADH: a retrospective study of 23 cases. Transl. Cancer Res. 10(3), 1229–1237 (2021). https://doi.org/10.21037/tcr-20-2123.
J.G. Verbalis, S. Adler, R.W. Schrier, T. Berl, Q. Zhao, F.S. Czerwiec; SALT Investigators, Efficacy and safety of oral tolvaptan therapy in patients with the syndrome of inappropriate antidiuretic hormone secretion. Eur. J. Endocrinol. 164(5), 725–732 (2011). https://doi.org/10.1530/EJE-10-1078
C. Selmer, J.C. Madsen, C. Torp-Pedersen, G.H. Gislason, J. Faber, Hyponatremia, all-cause mortality, and risk of cancer diagnoses in the primary care setting: a large population study. Eur. J. Intern. Med. 36, 36–43 (2016). https://doi.org/10.1016/j.ejim.2016.07.028
R. Berardi, M. Santoni, T. Newsom-Davis, M. Caramanti, S. Rinaldi, M. Tiberi, F. Morgese, M. Torniai, M. Pistelli, A. Onofri, M. Bower, S. Cascinu, Hyponatremia normalization as an independent prognostic factor in patients with advanced non-small cell lung cancer treated with first-line therapy. Oncotarget 8(14), 23871–23879 (2017). https://doi.org/10.18632/oncotarget.13372
L.M. Castello, M. Baldrighi, A. Panizza, E. Bartoli, G.C. Avanzi, Efficacy and safety of two different tolvaptan doses in the treatment of hyponatremia in the emergency department. Intern. Emerg. Med. 12(7), 993–1001 (2017). https://doi.org/10.1007/s11739-016-1508-5
P. Tzoulis, J.A. Waung, E. Bagkeris, H. Carr, B. Khoo, M. Cohen, P.M. Bouloux, Real-life experience of tolvaptan use in the treatment of severe hyponatraemia due to syndrome of inappropriate antidiuretic hormone secretion. Clin. Endocrinol. 84(4), 620–626 (2016). https://doi.org/10.1111/cen.12943
M. Matsuzaki, M. Hori, T. Izumi, M. Fukunami; Tolvaptan Investigators, Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc. Drugs Ther. 25(Suppl 1), S33–S45 (2011). https://doi.org/10.1007/s10557-011-6304-x
O. Hansen, P. Sørensen, K.H. Hansen, The occurrence of hyponatremia in SCLC and the influence on prognosis: a retrospective study of 453 patients treated in a single institution in a 10-year period. Lung cancer (Amsterdam, Netherlands) 68(1), 111–114 (2010). https://doi.org/10.1016/j.lungcan.2009.05.015
J. Martin-Grace, M. Tomkins, M.W. O’Reilly, C.J. Thompson, M. Sherlock, Approach to the Patient: hyponatremia and the Syndrome of Inappropriate Antidiuresis (SIAD). J. Clin. Endocrinol. Metab. 107(8), 2362–2376 (2022). https://doi.org/10.1210/clinem/dgac245
Acknowledgements
This research was in part supported by funds of the University of Ferrara (FAR 2019, 2020, 2021), in cooperation with the LTTA of the University of Ferrara.
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
M.B. and L.A. performed the research, analyzed and interpretated the data and wrote the paper. I.G., M.R.A. and M.C.Z. critically revised the manuscript. Marta Bondanelli designed the study. All the authors gave final approval of this version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
M.B., L.A., I.G., M.C.Z. and M.R.A. have no conflict of interest.
Human studies and informed consent
Informed consent was obtained from all patients to be included in the study. Approval to conduct this human subject research was obtained from the Local Ethics Committee (protocol number CE-AVEC 697/2020/Oss/AOUFe). All procedures were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bondanelli, M., Aliberti, L., Gagliardi, I. et al. Long-term low-dose tolvaptan efficacy and safety in SIADH. Endocrine 82, 390–398 (2023). https://doi.org/10.1007/s12020-023-03457-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03457-w