Abstract
Purpose
To investigate whether maternal cigarette smoking during pregnancy is a risk factor for developing GDM.
Methods
MEDLINE, Scopus, CENTRAL and Google Scholar databases were searched from inception to December 2022 to identify eligible original articles. A systematic review and meta-analysis (weighted data, random-effects model) were performed. The primary outcome was the development of GDM in pregnant women. The results were expressed as odds ratios (OR) with 95% confidence interval (CI) (inverse variance method). Subgroup analysis was planned according to the maternal smoking status and GDM diagnostic criteria. Statistical heterogeneity was checked with the Chi-squared (Chi2) test and the I2 index was used to quantify it. The studies were evaluated for publication bias.
Results
Thirty-five studies, including 23,849,696 pregnant women, met the inclusion criteria. The pooled OR of smoking during pregnancy compared with non-smoking (never smokers and former smokers) was 1.06 (95% CI 0.95–1.19), p = 0.30; I2 = 90%; Chi2 = 344; df=34; p < 0.001. Subgroup analysis was performed according to the two-step Carpenter-Coustan diagnostic criteria, due to the high heterogeneity among the other applied methods. The pooled OR for the Carpenter-Coustan subgroup was 1.19 (95% CI 0.95–1.49), p = 0.12; I2 = 63%; Chi2 = 27; df=10; p < 0.002. Further subgroup analysis according to maternal smoking status was not performed due to missing data.
Conclusion
There is no evidence to support an association between maternal cigarette smoking during pregnancy and the risk for GDM. Universally accepted diagnostic criteria for GDM must be adopted to reduce heterogeneity and clarify the association between smoking and GDM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is a hyperglycemic disorder first recognised during the second or third trimester of pregnancy, with major perinatal complications and long-term metabolic risks for the mother and the offspring [1]. During the last decades, women have tended to give birth in more advanced age and be more overweight, and as a result GDM prevalence is on the rise [2]. There are several factors predisposing to GDM, with advanced maternal age ( ≥ 35 years), maternal obesity (body mass index ≥ 30 kg/m2) and history of GDM in previous pregnancy being the most prominent [3]. Moreover, weight gain during pregnancy, family history of diabetes, polycystic ovary syndrome (PCOS), ethnicity (Asian, Asian American, African American, Native American, Pacific Islander, Latin), and achievement of pregnancy with Assisted Reproductive Technologies (ART) have been associated with GDM development [4, 5]. Cigarette smoking is a considerable threat to public health in both developed and developing countries, associated with all the leading causes of death [6]. Smoking is a modifiable risk factor for major cardiovascular events (MACE), cancer, type 2 diabetes mellitus (T2DM), and chronic obstructive pulmonary disease (COPD) [7, 8]. According to a meta-analysis, the global prevalence of smoking during pregnancy is 1.7%, with the highest being in the European region (8.1%) [9]. Smoking during pregnancy is related to placenta abruption, placenta previa, stillbirth, preterm premature rupture of membranes (PPROM) preterm delivery, pre-eclampsia, intrauterine growth restriction (IUGR), and congenital anomalies [10,11,12]. Tobacco smoking alters placental immunoregulation and function, and damage the feto-maternal interface at a cellular level [13]. Although cigarette smoking is a devasting and potentially destructive habit for the growing fetus, it is unclear whether it predisposes the mother to GDM development [14]. Several studies indicate passive or “secondhand” smoking as a risk factor for GDM development [15,16,17]. Two systematic reviews and meta-analyses concluded that there is no association between maternal smoking during pregnancy and GDM [18, 19]. As many relevant original studies have been published since 2017, the topic is worth a meta-analysis update.
Methods
Search strategy
The present study was conducted according to the recommendations of PRISMA guidelines (Preferred Reporting Items for Systematic Review and Meta-Analyses) [20]. The study’s protocol was enrolled in PROSPERO (registration number: CRD42022378857). A literature search in MEDLINE (PubMed), Scopus (Elsevier), CENTRAL (Cochrane Central Register of Controlled Trials), and Google Scholar databases was performed independently by two researchers (KIA and EP) from inception to December 28th, 2022.
Eligibility criteria
Eligible for inclusion were considered all the original studies [observational (cohort, cross-sectional, case-control) or interventional (randomized controlled trials)] written in the English language assessing the association of maternal cigarette smoking during pregnancy with the risk of GDM development. Studies involving pregnant women with pre-existing type 1 or T2DM, non-pregnant populations, pediatric, adolescent, or male populations, as well letters to the editor, editorials, case reports and case series were excluded.
Outcomes
The primary outcome of interest was the development of GDM in pregnant women smoking during pregnancy. The first arm included the active smokers and the second the non-smokers (never-smokers and former smokers). Former smokers ceased smoking at the beginning of gestation.
Study selection
The study selection was performed by two researchers (KIA and EP) independently; disagreements were resolved with the contribution of a third researcher (SAP), leading to consensus. The study selection was guided by the eligibility criteria and outcome of interest. The identified studies were imported to a systematic review software platform (COVIDENCE). Their title and abstract were screened for relevance, and followingly the full texts of selected studies were screened further for the final selection. Finally, the references of selected articles were also screened for the detection of more eligible studies.
Data extraction
The data extraction was performed in a pre-designed Microsoft Excel® sheet by two researchers (KIA and EP) that collected data independently on the name of the first author, the paper’s title, the year of the paper’s publication, the number of participants, the study’s type, the journal of publication, the country of origin, the study’s duration, the primary outcome, the study’s conclusion, the number of women in each subgroup (never-smokers, former and current smokers), the number of GDM cases in each subgroup, the diagnostic method of GDM, the confounders adjusted in each study, and the study’s conclusion. Any disagreements were resolved by a third researcher (SAP).
Statistical analysis
Statistical analysis was performed using Review Manager (RevMan) software (version 5.4.1 for Mac OS), as recommended by the Cochrane Collaboration. Dichotomous data were analyzed using an odds ratio (OR) and a 95% confidence interval (CI), according to the Inverse Variance method. The level of significance was set at 5%. The data were combined using the random-effects model because of the high heterogeneity among the included studies. The presence of heterogeneity was checked with the Chi-squared (Chi2) test (p-value < 0.1) and was quantified with the I2 Higgins test (0–40% non-significant/low heterogeneity, 40–50% moderate heterogeneity, ≥50% significant/high heterogeneity) [21]. A sensitivity analysis was performed to explain the high heterogeneity among studies. By removing one study at a time and recalculating the OR, the effect of each study on the summary estimate was observed. In the primary analysis, the effect of smoking versus non-smoking (never-smokers and former smokers) during pregnancy was investigated. In the subgroup analysis, the effect of GDM diagnostic method on the main outcome was analyzed.
Risk of bias
The risk of bias was assessed by two independent researchers (KIA and EP) with the 9-star Newcastle-Ottawa Scale (NOS) for cohort and case control studies (1–5 low quality, 6–9 high quality), and with the revised Joanna Briggs Institute (JBI) scale for cross-sectional studies (1–5 low quality, 6–8 high quality) [22, 23]. Publication bias was assessed with the construction of a funnel plot [24].
Results
Study selection
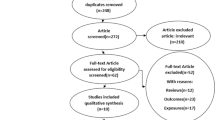
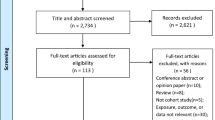
With the initial search 2,574 articles were retrieved. After title and abstract screening, 243 studies remained for full text assessment. Finally, 35 articles were included in the present systematic review and meta-analysis. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flowchart of selected studies is presented in Fig. 1.
Study characteristics
The characteristics of the included studies, published between 1992 and 2022, are summarized in Table 1.
Data synthesis (meta-analysis)
The results of the meta-analysis performed are summarized in Fig. 2.
GDM in smokers vs. non-smokers
OR 1.06 (95% CI 0.95–1.19, p = 0.30), indicating no significant association between cigarette smoking during pregnancy and GDM development. There was high heterogeneity (I2 = 90%; Chi2 = 344; df = 34; p < 0.001).
Subgroup analysis
Due to the high heterogeneity in the definition of GDM diagnostic methods, we proceeded to subgroup analysis of the studies using the two-step Carpenter-Coustan criteria. The results of subgroup analysis (Fig. 3) did not show an increase in the odds of GDM development in women smoking during pregnancy (OR 1.19, 95% CI 0.95–1.49, p = 0.12; I2 = 63%; Chi2 = 27; df = 10; p < 0.002) [25]. The heterogeneity was reduced in this subgroup analysis.
Additionally, we performed further subgroup analysis between the studies that provided: adjusted OR (Fig. 5), unadjusted OR (Fig. 6). Moreover, we performed subgroup analysis between the studies that provided adjusted OR and had considered similar confounders, such as: maternal age (Fig. 7), and BMI (Fig. 8). The results were similar with our initial analysis (OR ranging between 0.96–1.12), indicating that the unadjusted results and the various confounders did not distort our final conclusion.
Sensitivity analysis
High heterogeneity was observed. Removing each study, no significant changes in the effect estimate and I2 index were observed.
Risk of bias
The quality scores using NOS and the revised JBI scales are summarized in Table 1. Most of the studies were of high quality, and the quality scores ranged from 4 to 8 stars (5–8 for cohort and case-control studies, and 4–7 for cross-sectional). The main reason for downgrading the quality score was missing information on maternal smoking exposure. Publication bias was assessed with the funnel plot presented in Fig. 4. The funnel plot was symmetrical indicating low publication bias.
Discussion
The present systematic review and meta-analysis aimed to investigate the association between maternal cigarette smoking during pregnancy and the risk for GDM development. There is limited epidemiologic evidence supporting cigarette smoking as a risk factor for GDM. The present data synthesis of 35 studies concluded that maternal smoking during pregnancy is not associated with an increased risk for GDM (OR 1.06, 95% CI 0.95–1.19), a result consistent with the findings of the previous meta-analyses. To our knowledge, this is the third systematic review and meta-analysis (SRMA) on the topic, as there are two SRMAs published in 2008 and 2018 [18, 19]. Wendland et al. published the first relevant systematic review, including 12 studies, and concluded that there was no association between smoking during pregnancy and GDM (OR 1.03, 99% CI 0.85–1.25) [18]. Wang et al. meta-analysis, including 12 studies, found that there was no significant association between cigarette smoking during pregnancy and GDM development (OR 0.98, 95% CI 0.88–1.10) [19].
The present study is a meta-analysis of observational studies, and the potential confounders were acknowledged in Table 1. Results were primarily adjusted for the following confounders: maternal age, BMI, gestational weight gain, parity, race/ethnicity, GDM in a previous pregnancy, hypertension, education, and smoking status. While there was high heterogeneity between studies, sensitivity analysis did not show any influence of a single study on the overall result.
Among the including studies, there were controversial results about the smoking effect on GDM development. Fourteen studies showed an association between smoking during pregnancy and GDM, while 21 studies did not reveal any association (Table 1). However, only 17 studies had significant results.
In the subgroup analysis, an important reduction of heterogeneity was observed when two-step Carpenter-Coustan criteria were used for GDM diagnosis. The wide variations in GDM diagnostic methods and the need for universal GDM screening of pregnant women according to official guidelines are issues that should be urgently resolved.
The HAPO study (Hyperglycemia and Adverse Pregnancy Outcomes) altered the diagnostic approach of hyperglycemia in pregnancy [26]. Followingly, the International Association of Diabetes and Pregnancy Subgroup (IADPSG) established the 2-h 75-g OGTT, a detection strategy that increased the diagnostic prevalence of GDM, to protect mothers and fetuses from the devastating implications of overt hyperglycemia [12, 27].
As tobacco smoking during pregnancy may have detrimental implications for mother and fetus, women are encouraged to avoid it. Nevertheless, the actual mechanisms involved in the association of smoking and GDM manifestation need clarification. Nicotine increases cortisol, catecholamines and growth hormone secretion, hormones that counteract the actions of insulin, contributing to insulin resistance [28]. Pregnancy is a state of increased insulin resistance, owing to placental hormones like the human placental lactogen (hPL) and placental growth hormone, that support the growing fetus [29].
Although women are strongly encouraged to cease smoking during gestation, it was unexpected that some studies showed an association between smoking cessation and increased risk for GDM. A possible cause is that smoking cessation leads to increased caloric consumption and weight gain, a risk factor for GDM. Moreover, changes in adipocytes metabolism and cumulative exposure to smoking before cessation are associated with a greater risk for GDM in women who cease smoking upon gestation [30]. In the present study, we did not proceed to subgroup analysis between smokers and former smokers due to a lack of data.
Smoking results in a toxic environment of inflammatory and oxidative stress, undermining the health of mothers and fetuses. Although policies have been adopted for the prevention of active maternal smoking during pregnancy, exposure to tobacco through passive smoking from partners or/and family remains a threat to pregnant women [15]. The exposure of pregnant women to tobacco might be underestimated, as passive smoking is underreported (high recall and reporting bias); only a limited number of studies consider its impact on maternal outcomes. Passive smoking is an independent risk factor for GDM development in nulliparous women highlighting the need for prompt and intensive health education of pregnant women and their partners/families [15,16,17]. Preventive strategies should be implemented. In the present study, passive smoking was not independently evaluated, as none of the included studies [except for three (Hosler 2011, Xu 2017, Varela 2022)] provided relevant data.
The current study presents several strengths and certain limitations. Regarding strengths, the meta-analysis included 23,849,696 subjects and 35 studies published between 1992–2022 and conducted in 16 countries from almost every continent (e.g., USA, Canada, Brazil, Sweden, France, Italy, India, Korea, China), covering a representative population. Moreover, it is the third systematic review and meta-analysis evaluating the association between maternal cigarette smoking and the risk of GDM. Nine original studies were published since 2018 and incorporated in the present meta-analysis, along with several older studies.
In terms of limitations, the present systematic review and meta-analysis consists of 35 observational studies, as they were only available in the literature. The only study type that can prove a causation between a risk factor and a certain condition is the randomized controlled trial; nevertheless, it is unethical to conduct a trial with smoking intervention in pregnant women, as it would pose mothers and fetuses at a definitive risk. In all meta-analyses of observational studies, the confounding factors have to be identified and considered for a safe conclusion to be reached. Unfortunately, many of the included studies did not assess the potential confounders and presented unadjusted data, while the corresponding authors did not respond to our request for providing further data. However, subgroup analyses to evaluate the effect of unadjusted results on the final conclusion were conducted; the pooled outcome remained unchanged. Another limitation is that subgroup analysis according to tobacco exposure levels and smoking status was not performed, due to a lack of relevant data. Finally, there was high heterogeneity among the studies, which could be attributed to major differences in participants’ demographic characteristics, smoking assessment, and GDM diagnostic methods.
In conclusion, there is no evidence to suggest an association between maternal cigarette smoking during pregnancy and the development of GDM. However, further studies evaluating the impact of passive smoking and other potential confounding factors on GDM development should be performed. It is imperative, though, that the exposure of pregnant women to cigarette smoke should be limited even from the preconception period through investment in awareness policies, as it creates a toxic environment for the fetomaternal unit. GDM diagnostic criteria deserve a global consensus to establish a common ground on early identification and treatment.
References
R. Retnakaran, Diabetes in pregnancy 100 years after the discovery of insulin: Hot topics and open questions to be addressed in the coming years. Metabolism 119, 154772 (2021). https://doi.org/10.1016/j.metabol.2021.154772
Y. Zhang, C.M. Xiao, Y. Zhang, Q. Chen, X.Q. Zhang, X.F. Li et al. Factors associated with gestational diabetes mellitus: a meta-analysis. J. Diabetes Res 2021, 669–695 (2021). https://doi.org/10.1155/2021/6692695
L.C. Mendoza, J. Harreiter, D. Simmons, G. Desoye, J.M. Adelantado, F. Juarez et al. Risk factors for hyperglycemia in pregnancy in the DALI study differ by period of pregnancy and OGTT time point. Eur. J. Endocrinol 179, 39–49 (2018). https://doi.org/10.1530/EJE-18-0003
E. Hanson, I. Ringmets, A. Kirss, M. Laan, K. Rull, Screening of gestational diabetes and its risk factors: pregnancy outcome of women with gestational diabetes risk factors according to glycose tolerance test results. J. Clin. Med 11, 4953 (2022). https://doi.org/10.3390/jcm11174953
J.K. Bosdou, P. Anagnostis, D.G. Goulis, G.T. Lainas, B.C. Tarlatzis, G.F. Grimbizis et al. Risk of gestational diabetes mellitus in women achieving singleton pregnancy spontaneously or after ART: A systematic review and meta-analysis. Hum. Reprod. Update 26, 514–544 (2020). https://doi.org/10.1093/humupd/dmaa011
B. Thomson, J. Emberson, B. Lacey, S. Lewington, R. Peto, A. Jemal et al. Association between smoking, smoking cessation, and mortality by race, ethnicity, and sex among US Adults. JAMA Netw. Open 5, e2231480 (2022). https://doi.org/10.1001/jamanetworkopen.2022.31480
L.X. Yang, Z.J. Wang, D.M. Shi, M. Chai, L. Zhang, W.J. Cheng et al. Differential impact of cigarette smoking on prognosis in women and men undergoing percutaneous coronary intervention. Angiology 71, 281–287 (2020). https://doi.org/10.1177/0003319719889276
L. Zuo, F. He, G.G. Sergakis, M.S. Koozehchian, J.N. Stimpfl, Y. Rong et al. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol.- Lung Cell Mol. Physiol 307, 205–218 (2014). https://doi.org/10.1152/ajplung.00330.2013
S. Lange, C. Probst, J. Rehm, S. Popova, National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob. Heal 6, e769–e776 (2018). https://doi.org/10.1016/S2214-109X(18)30223-7
R.L. Andres, The association of cigarette smoking with placenta previa and abruptio placentae. Semin. Perinatol. 20, 154–159 (1996). https://doi.org/10.1016/S0146-0005(96)80083-8
A. Castles, E.K. Adams, C.L. Melvin, C. Kelsch, M.L. Boulton, Effects of smoking during pregnancy. Five meta-analyses. Am. J. Prev. Med 16, 208–215 (1999). https://doi.org/10.1016/S0749-3797(98)00089-0
E.M. Wendland, M.R. Torloni, M. Falavigna, J. Trujillo, M.A. Dode, M.A. Campos et al. Gestational diabetes and pregnancy outcomes - a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 14, 12–23 (2012). https://doi.org/10.1186/1471-2393-12-23
D.M. Morales-Prieto, P. Fuentes-Zacarías, J.M. Murrieta-Coxca, R.N. Gutierrez-Samudio, R.R. Favaro, J.S. Fitzgerald et al. Smoking for two- effects of tobacco consumption on placenta. Mol. Aspects Med. 87, 101023 (2022). https://doi.org/10.1016/j.mam.2021.101023
J. Maddatu, E. Anderson-Baucum, C. Evans-Molina, Smoking and the Risk of Type 2 Diabetes. Physiol. Behav 176, 139–148 (2017). https://doi.org/10.1016/j.trsl.2017.02.004
H.C. Lu, L. Yuan, H. Yu, H.S. Tang, Y.Y. Zhao, L. Huang et al. Passive smoking at home increased the risk of gestational diabetes mellitus in China. J. Public Heal 27, 733–741 (2019). https://doi.org/10.1007/s10389-018-1002-9
M. Morales-Suárez-Varela, I. Peraita-Costa, A. Perales-Marín, A. Llopis-Morales, A. Llopis-González, Risk of gestational diabetes due to maternal and partner smoking. Int. J. Environ. Res. Public Health 19, 925 (2022). https://doi.org/10.3390/ijerph19020925
J. Na, H. Chen, H. An, M. Ren, X. Jia, B. Wang et al. Passive smoking and risk of gestational diabetes mellitus among nonsmoking women: a prospective cohort study in China. Int. J. Environ. Res. Public Health 19, 4712 (2022). https://doi.org/10.3390/ijerph19084712
E.M. Wendland, M.E. Pinto, B.B. Duncan, J.M. Belizán, M.I. Schmidt, Cigarette smoking and risk of gestational diabetes: A systematic review of observational studies. BMC Pregnancy Childbirth 8, 1–8 (2008). https://doi.org/10.1186/1471-2393-8-53
J.W. Wang, S.S. Cao, R.Y. Hu, M. Wang, Association between cigarette smoking during pregnancy and gestational diabetes mellitus: a meta-analysis. J. Matern. Neonatal Med 33, 758–767 (2018). https://doi.org/10.1080/14767058.2018.1500547
M.J. Page, D. Moher, P.M. Bossuyt, I. Boutron, T.C. Hoffmann, C.D. Mulrow et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 372, 160 (2021). https://doi.org/10.1136/bmj.n160
J.P.T. Higgins, S.G. Thompson, J.J. Deeks, D.G. Altman, Measuring inconsistency in meta-analyses. Br. Med. J 327, 557–560 (2003). https://doi.org/10.1136/bmj.327.7414.557
Wells, G., Shea, B., Robertson, J., Peterson, J., Welch, V., Losos, M.: The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta- Analysis Bias and Confounding Newcastle-Ottowa Scale. Ottawa Hosp. Res. Inst. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2012). Accessed 5 January 2012
Moola, S., Munn, Z., Tufanaru, C., Aromataris, E.: Checklist for analytical cross sectional studies. Joanna Briggs Inst Rev Man. http://joannabriggs.org/research/critical-appraisal-tools.html (2017) Accessed 30 August 2017
C. Godavitarne, A. Robertson, D.M. Ricketts, B.A. Rogers, Understanding and interpreting funnel plots for the clinician. Br J. Hosp. Med 79, 578–583 (2018). https://doi.org/10.12968/hmed.2018.79.10.578
M.W. Carpenter, D.R. Coustan, Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol 144, 768–773 (1982). https://doi.org/10.1016/0002-9378(82)90349-0
The HAPO Study Cooperative Research Group*, Hyperglycemia and Adverse Pregnancy Outcomes. N. Engl. J. Med 358, 1991–2002 (2008). https://doi.org/10.1056/NEJMoa0707943
International Association of Diabetes and Pregnancy Study Groups Consensus Panel., International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682 (2010). https://doi.org/10.2337/dc09-1848
V. Durlach, B. Vergès, A. Al-Salameh, T. Bahougne, F. Benzerouk, I. Berlin et al. Smoking and diabetes interplay: A comprehensive review and joint statement. Diabetes Metab 48, 101370 (2022). https://doi.org/10.1016/j.diabet.2022.101370
L.T. Dickens, C.C. Thomas, Updates in Gestational Diabetes Prevalence, Treatment, and Health Policy. Curr. Diab. Rep. 19, 33 (2019). https://doi.org/10.1007/s11892-019-1147-0
C.M. Ferrara, M. Kumar, B. Nicklas, S. McCrone, A.P. Goldberg, Weight gain and adipose tissue metabolism after smoking cessation in women. Int. J. Obes 25, 1322–1326 (2001). https://doi.org/10.1038/sj.ijo.0801716
I. Feferkorn, A. Badeghiesh, G. Mills, H. Baghlaf, M. Dahan, The effects of smoking on pregnancy risks in women with polycystic ovary syndrome: A population-based study. Hum. Reprod 36, 2549–2557 (2021). https://doi.org/10.1093/humrep/deab145
K.S. Kim, S. Hong, K. Han, C.Y. Park, The Clinical Characteristics of Gestational Diabetes Mellitus in Korea: A National Health Information Database Study. Endocrinol. Metab. 36, 628–636 (2021). https://doi.org/10.3803/ENM.2020.948
S. Chanda, V. Dogra, N. Hazarika, H. Bambrah, A.K. Sudke, A. Vig et al. Prevalence and predictors of gestational diabetes mellitus in rural Assam: A cross-sectional study using mobile medical units. BMJ Open 10, e037836 (2020). https://doi.org/10.1136/bmjopen-2020-037836
S. Masalin, H. Kautiainen, M. Gissler, P. Pennanen, J.G. Eriksson, M.K. Laine, Impact of smoking on gestational diabetes mellitus and offspring birthweight in primiparous women. Acta Obstet. Gynecol. Scand. 99, 1632–1639 (2020). https://doi.org/10.1111/aogs.13924
Y. Bar-Zeev, Z.T. Haile, I.A. Chertok, Association between Prenatal Smoking and Gestational Diabetes Mellitus. Obstet. Gynecol 135, 91–99 (2020). https://doi.org/10.1097/AOG.0000000000003602
P. Konstantakou, S.A. Paschou, I. Patinioti, E. Vogiatzi, V. Sarantopoulou, E. Anastasiou, The effect of smoking on the risk of gestational diabetes mellitus and the OGTT profile during pregnancy. Diabetes Res. Clin. Pract. 158, 107901 (2019). https://doi.org/10.1016/j.diabres.2019.107901
M.L. Garmendia, S. Mondschein, B. Montiel, J.P. Kusanovic, Trends and predictors of gestational diabetes mellitus in Chile. Int. J. Gynecol. Obstet 148, 210–218 (2020). https://doi.org/10.1002/ijgo.13023
A. Sirico, A. Lanzone, I. Mappa, L. Sarno, M. Słodki, D. Pitocco et al. The role of first trimester fetal heart rate in the prediction of gestational diabetes: A multicenter study. Eur. J. Obstet. Gynecol. Reprod. Biol 243, 158–161 (2019). https://doi.org/10.1016/j.ejogrb.2019.10.019
S.E. Badon, D.A. Enquobahrie, P.D. Wartko, R.S. Miller, B. Gelaye, T.K. Sorensen et al. Healthy lifestyle during early pregnancy and risk of gestational diabetes mellitus. Am. J. Epidemiol 186, 326–333 (2017). https://doi.org/10.1093/aje/kwx095
A. Collier, E.C. Abraham, J. Armstrong, J. Godwin, K. Monteath, R. Lindsay, Reported prevalence of gestational diabetes in Scotland: The relationship with obesity, age, socioeconomic status, smoking and macrosomia, and how many are we missing? J. Diabetes Investig 8, 161–167 (2017). https://doi.org/10.1111/jdi.12552
X. Xu, Y. Liu, D. Liu, X. Li, Y. Rao, M. Sharma et al. Prevalence and determinants of gestational diabetes mellitus: A cross-sectional study in China. Int. J. Environ. Res. Public Health 14, 1–13 (2017). https://doi.org/10.3390/ijerph14121532
C. Erem, U.B. Kuzu, O. Deger, G. Can, Prevalence of gestational diabetes mellitus and associated risk factors in Turkish women: The Trabzon GDM Study. Arch. Med. Sci 11, 724–735 (2015). https://doi.org/10.5114/aoms.2015.53291
T.A. Moore Simas, K.L. Szegda, X. Liao, P. Pekow, G. Markenson, L. Chasan-Taber, Cigarette smoking and gestational diabetes mellitus in Hispanic woman. Diabetes Res. Clin. Pract. 105, 126–134 (2014). https://doi.org/10.1016/j.diabres.2014.04.026
C. Huy, A. Loerbroks, A. Hornemann, S. Röhrig, S. Schneider, Prevalence, trend and determining factors of gestational diabetes in Germany. Geburtshilfe Frauenheilkd 72, 311–315 (2012). https://doi.org/10.1055/s-0031-1298390
Y.T. Lagerros, S. Cnattingius, F. Granath, U. Hanson, A.K. Wikström, From infancy to pregnancy: Birth weight, body mass index, and the risk of gestational diabetes. Eur. J. Epidemiol 27, 799–805 (2012). https://doi.org/10.1007/s10654-012-9721-7
A.S. Hosler, S.G. Nayak, A.M. Radigan, Stressful events, smoking exposure and other maternal risk factors associated with gestational diabetes mellitus. Paediatr. Perinat. Epidemiol 25, 566–574 (2011). https://doi.org/10.1111/j.1365-3016.2011.01221.x
A.E. Haskins, E.R. Bertone-Johnson, P. Pekow, E. Carbone, R.T. Fortner, L. Chasan-Taber, Smoking during pregnancy and risk of abnormal glucose tolerance: a prospective cohort study. BMC Pregnancy Childbirth 10, 55–63 (2010). https://doi.org/10.1186/1471-2393-10-55
J. Roelands, M.G. Jamison, A.D. Lyerly, A.H. James, Consequences of smoking during pregnancy on maternal health. J. Women’s Heal 18, 867–872 (2009). https://doi.org/10.1089/jwh.2008.1024
J.A. Rauh-Hain, S. Rana, H. Tamez, A. Wang, B. Cohen, A. Cohen et al. Risk for developing gestational diabetes in women with twin pregnancies. J. Matern. Neonatal Med 22, 293–299 (2009). https://doi.org/10.1080/14767050802663194
J.S. Radesky, E. Oken, S.L. Rifas-Shiman, K.P. Kleinman, J.W. Rich-Edwards, M.W. Gillman, Diet during early pregnancy and development of gestational diabetes. Paediatr. Perinat. Epidemiol 22, 47–59 (2008). https://doi.org/10.1111/j.1365-3016.2007.00899.x
E.M. Wendland, B.B. Duncan, J.M. Belizan, A. Vigo, M.I. Schmidt, Gestational diabetes and pre-eclampsia: Common antecedents. Arq. Bras. Endocrinol. Metabol. 52, 975–984 (2008). https://doi.org/10.1590/S0004-27302008000600008
E. Cosson, M. Benchimol, L. Carbillon, I. Pharisien, J. Pariès, P. Valensi et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab 32, 140–146 (2006). https://doi.org/10.1016/S1262-3636(07)70260-4
I. Östlund, B. Haglund, U. Hanson, Gestational diabetes and preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol 113, 12–16 (2004). https://doi.org/10.1016/j.ejogrb.2003.07.001
L.J. England, R.J. Levine, C. Qian, L.M. Soule, E.F. Schisterman, K.F. Yu et al. Glucose tolerance and risk of gestational diabetes mellitus in nulliparous women who smoke during pregnancy. Am. J. Epidemiol 160, 1205–1213 (2004). https://doi.org/10.1093/aje/kwh340
D.P. Terry, E. Weiderpass, C.-G. Ostenson, S. Cnattingius, Cigarette Smoking and the Risk of Gestational and Pregestational Diabetes. Diabetes Care 26, 2994–2998 (2003). https://doi.org/10.2337/diacare.26.11.2994
M. Wolf, L. Sandler, K. Hsu, K. Vossen-Smirnakis, J.L. Ecker, R. Thadhani, First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care 26, 819–824 (2003). https://doi.org/10.2337/diacare.26.3.819
X. Yang, B. Hsu-Hage, H. Zuang, L. Yu, L. Dong, J. Li et al. Gestational diabetes mellitus in women of single gravidity in Tianjin City, China. Diabetes Care 25, 847–851 (2002). https://doi.org/10.2337/diacare.25.5.847
K.E. Innes, T.E. Byers, J.A. Marshall, A. Barón, M. Orleans, R.F. Hamman, Association of a woman’s own birth weight with subsequent risk for gestational diabetes. JAMA 287, 2534–2541 (2002). https://doi.org/10.1001/jama.287.19.2534
S. Bo, G. Menato, A. Lezo, A. Signorile, C. Bardelli, F. De Michieli et al. Dietary fat and gestational hyperglycaemia. Diabetologia 44, 972–978 (2001). https://doi.org/10.1007/s001250100590
X. Xiong, L.D. Saunders, F.L. Wang, N.N. Demianczuk, Gestational diabetes mellitus: Prevalence, risk factors, maternal and infant outcomes. Int. J. Gynecol. Obstet 75, 221–228 (2001). https://doi.org/10.1016/S0020-7292(01)00496-9
B. Zarén, G. Lindmark, L. Wibell, I. Følling, The effect of smoking on glucose homeostasis and fetal growth in pregnant women. Ups. J. Med. Sci. 105, 41–56 (2000). https://doi.org/10.1517/03009734000000046
G.M. Joffe, J. Esterlitz, R.J. Levine, The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Am. J. Obstet. Gynecol 179, 1032–1037 (1998). https://doi.org/10.1016/s0002-9378(97)80411-5
C.G. Solomon, W.C. Willett, V.J. Carey, J. Rich-Edwards, D.J. Hunter, G.A. Colditz et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA 278, 1078–1083 (1997). https://doi.org/10.1001/jama.278.13.1078
G.S. Berkowitz, R.H. Lapinski, R. Wein, D. Lee, Race/ethnicity and other risk factors for gestational diabetes. Am. J. Epidemiol 135, 965–973 (1992). https://doi.org/10.1093/oxfordjournals.aje.a116408
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Kleoniki I. Athanasiadou, Stavroula A. Paschou, Evgenia Papakonstantinou, Vasiliki Vasileiou, and Fotini Kanouta. The first draft of the manuscript was written by Kleoniki I. Athanasiadou, Paraskevi Kazakou, and Katerina Stefanaki, and all authors commented on previous versions of the manuscript. Statistical analyses were performed by Kleoniki I. Athanasiadou and Evgenia Papakonstantinou. Risk of bias assessment was performed by Kleoniki I. Athanasiadou, Paraskevi Kazakou, and Theodora Psaltopoulou. Supervision was performed by Dimitrios G. Goulis, Georgia N. Kassi, and Eleni Anastasiou. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Athanasiadou, K.I., Paschou, S.A., Papakonstantinou, E. et al. Smoking during pregnancy and gestational diabetes mellitus: a systematic review and meta-analysis. Endocrine 82, 250–262 (2023). https://doi.org/10.1007/s12020-023-03423-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03423-6