Abstract
Purpose
To retrospectively summarize the clinical features of acromegaly complicated with fulminant pituitary apoplexy and analyze the prognostic factors to guide early identification and timely treatment of such patients.
Methods
A retrospective analysis was carried out to summarize the clinical manifestations, hormone changes, imaging, treatment and follow-up of ten patients with acromegaly complicated with fulminant pituitary apoplexy admitted to our hospital from February 2013 to September 2021.
Results
The mean age of the ten patients (five males and five females) at the time of pituitary apoplexy was 37.1 ± 13.4 years old. There were nine cases with sudden severe headaches and five cases with visual impairment. All patients had pituitary macroadenomas, of which six cases with Knosp grade ≥3. The level of GH/IGF-1 hormone after pituitary apoplexy was lower compared with pre-apoplexy, and 1 patient reached biochemical remission spontaneously. Seven patients underwent transsphenoidal pituitary surgery after apoplexy and one patient was treated with long-acting somatostatin analog. The biochemical remission rate was 37.5% in eight patients immediately after treatment and 50% at the last follow-up. Patients with Knosp grade ≥3 were less likely to achieve biochemical remission than those with Knosp grade <3 (16.7% vs. 100%, p = 0.048), and patients who achieved biochemical remission had a smaller maximum tumor diameter [20.1 (20.1,28.0) mm vs. 44.0 (44.0,60) mm, p = 0.016].
Conclusion
Acromegaly complicated with fulminant pituitary apoplexy remains a diagnostic and therapeutic challenge.
Similar content being viewed by others
Introduction
Acromegaly is caused by the overproduction of growth hormone (GH) that stimulates the over synthesis of insulin-like growth factor 1 (IGF-1), resulting in excessive hyperplasia of soft tissue, bone, and cartilage throughout the body, which can lead to multi-organ system complications such as cardiovascular system, respiratory system, and metabolic abnormalities. According to the latest meta-analysis, the prevalence of acromegaly was 5.9 (95% CI: 4.4–7.9) per 100,000 persons [1]. More than 95% of acromegaly is caused by pituitary GH-secreting adenomas [2]. Pituitary apoplexy, defined as infarction or hemorrhage of the pituitary gland, mostly occurs in pituitary adenomas, and is often associated with headache, visual dysfunction, and hypopituitarism. The incidence of pituitary adenomas complicated with apoplexy has been reported to be 0.6–13% [3]. Pituitary apoplexy can be classified as fulminant pituitary apoplexy (also known as classical pituitary apoplexy) and silent pituitary apoplexy (or subclinical pituitary apoplexy) according to clinical manifestations. The former is characterized by sudden severe headache or rapid vision loss, while the latter has no acute manifestations and is usually diagnosed by imaging, hormone measurements, or operation [4]. Management strategy of pituitary apoplexy decided by a multidisciplinary team is strongly recommended. Frequent monitoring and regular follow-ups are essential for determining the choice of surgery or conservative treatment. However, there is still a lack of clinical evidence on the best treatment option [5]. To date, there have been few reports of acromegaly complicated with fulminant pituitary apoplexy. To improve clinicians’ understanding of this clinical emergency, this study retrospectively summarized the clinical characteristics, treatment, and follow-up of ten patients diagnosed as acromegaly complicated with fulminant pituitary apoplexy in our hospital since 2013, and reviewed the relevant articles.
Patients and methods
Patient population
In this retrospective study, ten patients discharged from Peking Union Medical College Hospital (PUMCH) with the diagnosis of “acromegaly” combined with “pituitary apoplexy” from February 2013 to September 2021 were included. The inclusion criteria were: (1) meets the criteria of acromegaly, including clinical manifestations of acromegaly, IGF-1 higher than the upper limit of the age-sex-matched reference range, GH nadir ≥1.0 ng/ml during oral glucose tolerance test (OGTT), and pituitary adenoma diagnosed on imaging [2]. (2) Manifests as sudden severe headache or acute vision loss. (3) Pituitary hemorrhage or coagulative necrosis shown in imaging, operation, or pathology.
Evaluation of biochemical remission
The criteria for biochemical remission of acromegaly were as follow: random GH or GH nadir during OGTT < 1.0 ng/ml, along with IGF-1 decreased to the normal range. In this article, the relative level of IGF-1 was described according to upper limit of normal (ULN) based on the reference range matched by age and sex.
Follow up visits
Patients were followed up for 3 months to 9 years after treatment, with a mean follow-up of 3 years. Eight patients were followed up for more than 1 year. The last follow-up biochemical remission was defined as random GH, GH nadir during OGTT, and IGF-1 meeting the above criteria at the last visit in our hospital or other hospitals.
Statistical analysis
The statistical and analytical procedures were performed using SPSS software version 25.0. Continuous variables were compared by t-test or Wilcoxon rank sum test, according to Kolmogorov-Smirnov normality evaluation. Correlations were presented with Pearson or Spearman correlation coefficient. Categorical variables were analyzed by Chi-square test or Fisher exact test. Statistical significance was defined as p < 0.05.
Results
Characterization of the study population
A total of 1850 acromegaly patients were admitted to our hospital from February 2013 to September 2021, of which 10 patients were diagnosed with fulminant pituitary apoplexy, with an incidence of 0.54%. The ratio of male to female was 1:1 and the mean age at apoplexy was 37.1 ± 13.4 years old. The average course from acromegaly to apoplexy was 6.8 ± 5.8 years. Eight patients visited a hospital within 2 weeks from the onset of apoplexy, one in 2 months after severe headache, and 1 in 9 months after acute vision loss in one eye. Six patients had multiple comorbidities, including five cases of diabetes mellitus, three cases of hypertension, one case of hyperlipidemia, and one case of coronary heart disease.
Clinical manifestations of pituitary apoplexy
Among the ten patients, nine cases had sudden severe headache, including eight cases with vomiting, four cases with vision loss, and two cases with diplopia. One patient only presented as acute loss of vision. In addition, two patients developed drowsiness during the apoplexy, and one had fever with a maximum temperature of 38.5 °C and neck resistance. After treatment, the above symptoms were all relieved except one patient who was admitted to hospital 9 months after apoplexy failed to recover her vision.
Changes in hormone levels before and after pituitary apoplexy
Changes in GH/IGF-1 levels
The median random GH after pituitary apoplexy in ten patients was 25.5 (12.63, 46.33) ng/ml. The average GH nadir and IGF-1×ULN were 14.84 ± 9.87 ng/ml and 1.84 ± 1.13. GH/IGF-1 levels were recorded in four patients before and after apoplexy (Table 1), with average decreases of 85.3% and 43.1% in random GH and absolute IGF-1 values after the onset. One patient reached biochemical remission at 2 months after apoplexy. The variation trends of GH/IGF-1 were recorded in three patients within 3 weeks after apoplexy. Random GH, OGTT-GH nadir, and IGF-1 levels decreased gradually with time, but none of them fell back to normal value.
Changes in other pituitary functions
There were seven patients with secondary hypothyroidism, five with hypogonadotropic hypogonadism, five with secondary hypoadrenocorticism, and one with central diabetes insipidus. Among four patients with documented pre-apoplexy hormone levels, menstruation history, and medication, two developed new hypopituitarism after stroke. One case only developed secondary hypothyroidism, the other presented secondary hypothyroidism, secondary hypoadrenocorticism, and central diabetes insipidus simultaneously.
Pituitary magnetic resonance imaging (MRI) features
All ten patients had macroadenomas, with a median maximum diameter of 30.8 (24.6–41.0) mm from 15.8 mm to 60.0 mm, of which three were giant adenomas [6]. MRI of the pituitary gland was available in nine patients, including two, one, two, and four cases with Knosp grading of 1–4, respectively, and six of them presented invasiveness of cavernous sinus (Knsop ≥ 3). Two cases showed a slightly low signal on T1, and the others showed isointensity to hyperintensity on T1 and T2. After gadolinium injection, all cases showed uneven or rim enhancement and signs of hemorrhage or necrosis. (Fig. 1) Compression and elevation of optic chiasma were presented in four patients with visual impairment, and short T1 signal of posterior pituitary disappeared in one patient with central diabetes insipidus.
Typical pituitary MRI characteristics of acromegaly complicated with fulminant pituitary apoplexy (a) (d) (g) 7 days before apoplexy: macroadenoma about 26 × 19 × 20 mm showed isointensity on both T1 and T2 and mild enhancement; (b) (e) (h) 9 days after apoplexy: macroadenoma about 31 × 21 × 21 mm showed hyperintensity on both T1 and T2 and uneven enhancement, indicating subacute hemorrhage; (c) (f) (i) 3 months after surgery: postoperative changes in the pituitary gland, with isointensity to hyperintensity on T1, hypointensity to isointensity on T2, and mild enhancement
Coagulation measurements
Blood coagulation was measured in nine patients after apoplexy. The reference ranges of prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen (FBG) in PUMCH are 10.4–12.6 s, 28.9–32.5 s, and 1.8–3.5 g/L, respectively. Except for one patient who was using warfarin, the average PT and APTT were 12.61 ± 0.91 s and 33.48 ± 6.55 s respectively, and median FBG was 2.72 (2.35, 4.85) g/L. Further analysis showed a significant positive correlation between PT and IGF-1 × ULN (Fig. 2, Pearson correlation coefficient = 0.766, p = 0.027).
Treatment
Treatment methods
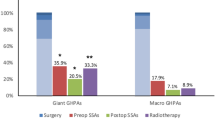
Among the ten patients, one patient went to our hospital for radiotherapy after going through surgery, radiotherapy, and medication since his onset of pituitary apoplexy 17 years ago; another patient achieved biochemical remission simultaneously 2 months after pituitary apoplexy and therefore did not receive further treatment. The other eight apoplexy cases were treated for the first time, of which seven cases received operation, and one case underwent intramuscular injection of 20 mg octreotide acetate microspheres due to high operation risk caused by dilated cardiomyopathy. Dark old blood or bean dregs-like necrosis could be seen during those seven operations. Postoperative histopathological analysis were obtained in five cases, three of which had Ki-67 ≥ 3%. Patients with hypopituitarism received physiological hormone replacement.
GH/IGF-1 changes after treatment
These eight patients had a significant decrease in random GH, OGTT-GH nadir and IGF-1 × ULN after treatment [Table 2, 24.05 ± 26.35 ng/ml vs. 1.65 (0.70, 5.03) ng/ml, p = 0.017; 13.93 ± 9.50 ng/ml vs. 2.00 (0.30, 5.33) ng/ml, p = 0.043; 1.65 ± 0.63 vs. 0.95 ± 0.40, p = 0.029]. Two patients treated with surgery and one patient treated with long-acting somatostatin analog achieved biochemical remission after treatment immediately; the other five patients who did not achieve biochemical remission experienced 54% [34.12 ± 29.12 ng/ml vs. 3.60 (1.50, 16.65) ng/ml] and 15% (1.53 ± 0.62 vs. 1.20 ± 0.24) decrease in average in random GH and IGF-1 × ULN, respectively after surgery.
Follow-up and prognosis
The overall remission rate at the last follow-up was 50% in ten patients. Tumor recurrence was confirmed by MRI in one case 5 years after operation, and the GH/IGF-1 hormone levels of other four cases showed a descending trend but did not reach biochemical remission up to the last follow-up. The remission rate was significantly higher in patients with Knosp grade <3 at the time of apoplexy than those with Knosp grade ≥ 3 (100% vs. 16.7%, p = 0.048). Also, patients who achieved remission during the follow-up had significantly smaller maximum diameter of the adenoma [20.1 (20.1, 28.0) mm vs. 44.0 (44.0, 60.0) mm, p = 0.016]. In addition, only one of the five patients with visual impairment at the onset achieved remission. Hypopituitarism that developed after apoplexy did not recover after the operation and during follow-up.
Comparison with previous literature
In 1986, our department summarized the clinical characteristics of eight cases of acromegaly complicated with fulminant pituitary apoplexy admitted in 1979–1984 in PUMCH [7]. We also performed a review of literature, searching on PubMed and CNKI using the keywords “pituitary apoplexy”, “pituitary infarction”, “acromegaly”, and “growth hormone adenoma”. We retrieved 109 articles related, including 75 cases of acromegaly complicated with fulminant pituitary apoplexy. In Table 3, we summarized all the cases above and compared with case series in PUMCH. The gender and age compositions were similar between three groups. In addition to typical headache and visual impairment, some patients also manifested other neurological symptoms such as fever, meningeal irritation, and consciousness disorders. Most of the patients presented a decreasing trend of GH/IGF-1 hormone after apoplexy. Imaging features showed that almost all the pituitary GH adenomas complicated with apoplexy were macroadenomas. MRI was the best way to diagnose pituitary lesions, while CT had satisfying performance in determining hemorrhage in emergency. Compared with the previous 8 patients in PUMCH, the incidence of pituitary apoplexy in acromegaly patients in this series was lower than before (0.54% vs. 2.4%, p = 0.003), and the time from onset to medical consultation was significantly shorter [0.50 (0.50, 0.88) months vs.1.50 (1.00, 11.25) months, p = 0.007].
Discussion
Pituitary apoplexy is an infrequent complication of acromegaly, in which fulminant pituitary apoplexy is characterized by acute onset and severe symptoms that even endanger patients’ lives [8]. Therefore, it is one of the most acute and severe diseases in neurosurgery and endocrinology department. We found that compared with the past, the admission of patients with pituitary apoplexy was more timely, and the proportion of fulminant pituitary apoplexy in acromegaly decreased significantly, which might be related to the increase in clinicians’ awareness of early identification and treatment of acromegaly in recent years.
Sudden headache or visual impairment are typical clinical manifestations of pituitary apoplexy. More attention should be paid to the occurrence of pituitary apoplexy especially for patients with acromegaly signs or medical history. In addition, pituitary apoplexy can also present as consciousness disorder, fever, neck resistance, and other manifestations, which need to be distinguished from neurological emergencies such as subarachnoid hemorrhage and meningoencephalitis.
At present, MRI is the first choice for the diagnosis of pituitary lesions. It was reported that 70.7% of the patients with acromegaly had macroadenomas, most of which were 11–20 mm, and 33.6% were invasive to the cavernous sinus [9]. In this study, all ten patients had pituitary macroadenomas, and 60% of which were invasive. Thus, macroadenomas and cavernous sinus invasion may be high risk factors for pituitary apoplexy in patients with acromegaly [8]. For patients with GH-secreting macroadenoma, it’s necessary to educate patients about the risk and typical clinical manifestations of pituitary apoplexy and timely admission to hospital once the related symptoms occur. Previous studies have reported that the risk factors of pituitary apoplexy include recent surgery (especially cardiac surgery), pregnancy, pituitary function stimulation test, use of cabergoline and bromocriptine, coagulation dysfunction, and a history of head trauma and radiotherapy [10]. In this study, six patients were combined with hypertension or diabetes. Although it has been suggested that hypertension and diabetes might impair microvascular function, a clear correlation between these comorbidities and pituitary apoplexy has not been confirmed [11].
For fulminant pituitary apoplexy, transsphenoidal resection of pituitary adenoma is still the main treatment. The hormone levels of some patients can gradually return to normal after radiotherapy, drug treatment, or observation. It has been reported that surgical or conservative treatment is similar in the recovery rate of visual impairment and cranial nerve function, so it is still controversial that which should be the first choice for fulminant pituitary apoplexy [3]. British guidelines recommended that patients with visual impairment or consciousness disorders after apoplexy should undergo surgery as soon as possible, while conservative treatment was recommended for patients without the above symptoms, especially those with high surgery risks [12]. Considering that more severe cases are more likely to take operations, it is difficult to avoid selection bias when comparing the prognosis of different treatment methods since the baseline conditions are different. Therefore, treatment strategy still needs to follow the principle of individualization [13]. In addition, there is still a lack of consensus and guideline for different types of pituitary adenomas complicated with fulminant pituitary apoplexy. The symptoms of headache and visual impairment were obviously relieved in all cases of pituitary GH-secreting macroadenoma provided here who received timely treatment. At present, the first-line treatment of pituitary GH-secreting macroadenomas is still surgery, and the remission rate of medication is only 19–60% [6, 14] Considering that the larger the tumor, the lesser the possibility of total tumor tissue necrosis after apoplexy, for patients with GH-secreting macroadenoma with pituitary apoplexy, especially for patients with occupying symptoms, the first choice of transsphenoidal resection should be recommended.
Compared with fulminant pituitary apoplexy, silent pituitary apoplexy may be more common [15]. Among the 40 patients diagnosed with “acromegaly” and “pituitary apoplexy” in PUMCH from 2013 to 2021, besides the 10 patients described above, 30 patients were silent pituitary apoplexy confirmed by imaging, surgery, and pathology. The clinical symptoms of acromegaly patients with silent pituitary apoplexy were milder and often atypical, which might include chronic headache or vision loss, fatigue, oligomenorrhea, and other manifestations of hypopituitarism. Generally, emergency surgery for decompression is not common, but surgical treatment is still the first choice for patients who do not reach biochemical remission after apoplexy.
GH/IGF-1 decreased in varying degrees in patients after apoplexy. Dozens of cases of spontaneous remission after pituitary apoplexy in acromegaly were reported in previous literature, but most of them lacked follow-up [16,17,18]. 1 case reached biochemical remission of acromegaly after apoplexy, but relapsed 18 years later, suggesting that the pituitary adenoma after apoplexy still has the risk of recurrence and needs long-term regular follow-up and monitoring [19]. Previous study reported that the lower the GH level of acromegaly patients before treatment, the better the prognosis [20]. But due to the different degrees of damage to pituitary function after apoplexy, this pattern was not found in our study.
In addition, this study found that there may be a positive correlation between PT and the relative level of IGF-1 after pituitary apoplexy. However, we did not find similar reports in previous studies, more evidence supported that acromegaly patients often showed hypercoagulable state, and the increase of plasma fibrinogen level is positively correlated with IGF-1 [21]. Some studies have found that antithrombin III was significantly increased in patients with active acromegaly, which could inhibit the effects of thrombin and activate coagulation factors VIIa, IXa, Xa, XIa and XIIa [22]. Whether the levels of PT and APTT in acromegaly patients are related to IGF-1, and whether there are abnormalities in coagulation function in patients with pituitary apoplexy need to be further studied.
The main limitation of our study is the small sample size since this is a single-center study, and fulminant pituitary apoplexy is not common in acromegaly. Second, since this is retrospective research, the clinical data are not complete, especially for the GH/IGF-1 level, other pituitary functions, and imaging data before apoplexy. Third, influenced by the COVID-19 pandemic, some patients failed to follow up regularly, which might affect the results of prognosis.
In conclusion, this study summarized ten cases of acromegaly complicated with fulminant pituitary apoplexy in PUMCH in recent years and reviewed the cases from previous literature for the first time. Fulminant pituitary apoplexy usually occurred in large and invasive pituitary GH-secreting adenomas with typical clinical manifestations. The prognosis was related to tumor size and invasiveness. It is necessary to identify this disease early and improve the long-term prognosis of patients through timely treatment and standardized follow-up.
References
S. Crisafulli, N. Luxi, J. Sultana, A. Fontana, F. Spagnolo, G. Giuffrida, F. Ferraù, D. Gianfrilli, A. Cozzolino, M. Cristina De Martino, F. Gatto, F. Barone-Adesi, S. Cannavò, G. Trifirò, Global epidemiology of acromegaly: a systematic review and meta-analysis. Eur. J. Endocrinol. 185(2), 251–263 (2021). https://doi.org/10.1530/eje-21-0216
C.P.A.C. Group, Chinese consensus on diagnosis and treatment of acromegaly (2021 version). Natl Med. J. China 101(27), 2115–2126 (2021). https://doi.org/10.3760/cma.j.cn112137-20210106-00022
J.P. Almeida, M.M. Sanchez, C. Karekezi, N. Warsi, R. Fernández-Gajardo, J. Panwar, A. Mansouri, S. Suppiah, F. Nassiri, R. Nejad, W. Kucharczyk, R. Ridout, A.F. Joaquim, F. Gentili, G. Zadeh, Pituitary apoplexy: results of surgical and conservative management clinical series and review of the literature. World Neurosurg. 130, e988–e999 (2019). https://doi.org/10.1016/j.wneu.2019.07.055
G. Li, Y. Shi, Pituitary apoplexy. Beijing Med. (S1), 60–63 (1986)
C. Capatina, W. Inder, N. Karavitaki, J.A. Wass, Management of endocrine disease: pituitary tumour apoplexy. Eur. J. Endocrinol. 172(5), R179–R190 (2015). https://doi.org/10.1530/eje-14-0794
P. Iglesias, V. Rodriguez Berrocal, J.J. Diez, Giant pituitary adenoma: histological types, clinical features and therapeutic approaches. Endocrine 61(3), 407–421 (2018). https://doi.org/10.1007/s12020-018-1645-x
Y. Shi, G. Li, C. Zeng, E. Hao, The change of clinical features and endocrine function of acromegaly patients with pituitary apoplexy. Chin. J. Endocrinol. Metab. 2(4), 213–216 (1986)
H. Zhang, K. Shu, F. Dong, H. Tang, L. Li, T. Lei, MRI and clinical features of pituitary apoplexy. J. Huazhong Univ. Sci. Technol. (Med. Sci.) 33(6), 749–751+759 (2004).
I. Potorac, P. Petrossians, A.F. Daly, F. Schillo, C. Ben Slama, S. Nagi, M. Sahnoun, T. Brue, N. Girard, P. Chanson, G. Nasser, P. Caron, F. Bonneville, G. Raverot, V. Lapras, F. Cotton, B. Delemer, B. Higel, A. Boulin, S. Gaillard, F. Luca, B. Goichot, J.L. Dietemann, A. Beckers, J.F. Bonneville, Pituitary MRI characteristics in 297 acromegaly patients based on T2-weighted sequences. Endocr. Relat. Cancer 22(2), 169–177 (2015). https://doi.org/10.1530/ERC-14-0305
P.L. Semple, J.A. Jane Jr, E.R. Laws Jr, Clinical relevance of precipitating factors in pituitary apoplexy. Neurosurg 61(5), 956–961 (2007). https://doi.org/10.1227/01.neu.0000303191.57178.2a. discussion 961-952
V. Biousse, N.J. Newman, N.M. Oyesiku, Precipitating factors in pituitary apoplexy. J. Neurol. Neurosurg. Psychiatry 71(4), 542–545 (2001). https://doi.org/10.1136/jnnp.71.4.542
S. Rajasekaran, M. Vanderpump, S. Baldeweg, W. Drake, N. Reddy, M. Lanyon, A. Markey, G. Plant, M. Powell, S. Sinha, J. Wass, UK guidelines for the management of pituitary apoplexy. Clin. Endocrinol. 74(1), 9–20 (2011). https://doi.org/10.1111/j.1365-2265.2010.03913.x
A. Cavalli, A. Martin, D.J. Connolly, S. Mirza, S. Sinha, Pituitary apoplexy: how to define safe boundaries of conservative management? Early and long-term outcomes from a single UK tertiary neurosurgical unit. Br. J. Neurosurg. 35(3), 334–340 (2021). https://doi.org/10.1080/02688697.2020.1812523
A. Micko, M.S. Agam, A. Brunswick, B.A. Strickland, M.J. Rutkowski, J.D. Carmichael, M.S. Shiroishi, G. Zada, E. Knosp, S. Wolfsberger, Treatment strategies for giant pituitary adenomas in the era of endoscopic transsphenoidal surgery: a multicenter series. J. Neurosurg. 136(3), 776–785 (2022). https://doi.org/10.3171/2021.1.JNS203982
A. Klimko, C. Capatina, Pituitary macroadenoma presenting as acromegaly and subacute pituitary apoplexy: case report and literature review. Cureus 12(8), e9612 (2020). https://doi.org/10.7759/cureus.9612
L.A. Fraser, D. Lee, P. Cooper, S. Van Uum, Remission of acromegaly after pituitary apoplexy: case report and review of literature. Endocr. Pract. 15(7), 725–731 (2009). https://doi.org/10.4158/EP09126.CRR
X.L. Wang, J.T. Dou, Z.H. Lü, W.W. Zhong, J.M. Ba, D. Jin, J.M. Lu, C.Y. Pan, Y.M. Mu, Spontaneous remission of acromegaly or gigantism due to subclinical apoplexy of pituitary growth hormone adenoma. Chin. Med. J. 124(22), 3820–3823 (2011)
R.C. Zhang, Y.F. Mu, J. Dong, X.Q. Lin, D.Q. Geng, Complex effects of apoplexy secondary to pituitary adenoma. Rev. Neurosci. 28(1), 59–64 (2017). https://doi.org/10.1515/revneuro-2016-0013
P.L. Werner, J.H. Shah, S.C. Kukreja, S.M. Miller, G.A. Williams, Recurrence of acromegaly after pituitary apoplexy. J. Am. Med Assoc. 247(20), 2816–2818 (1982)
A. Tomasik, M. Stelmachowska-Banaś, M. Maksymowicz, I. Czajka-Oraniec, D. Raczkiewicz, G. Zieliński, J. Kunicki, W. Zgliczyński, Clinical, hormonal and pathomorphological markers of somatotroph pituitary neuroendocrine tumors predicting the treatment outcome in acromegaly. Front Endocrinol. 13, 957301 (2022). https://doi.org/10.3389/fendo.2022.957301
A. Colak, H. Yilmaz, Y. Temel, M. Demirpence, N. Simsek, I. Karademirci, U. Bozkurt, E. Yasar, Coagulation parameters and platelet function analysis in patients with acromegaly. J. Endocrinol. Investig. 39(1), 97–101 (2016). https://doi.org/10.1007/s40618-015-0300-0
A. Amado, F. Araujo, D. Carvalho, Cardiovascular risk factors in acromegaly: what’s the impact of disease control. Exp. Clin. Endocrinol. Diabetes 126(8), 505–512 (2018). https://doi.org/10.1055/s-0043-124668
Funding
Provided by National High-Level Hospital Clinical Research Funding (2022-PUMCH-A-155) and National High-Level Hospital Clinical Research Funding (2022-PUMCH-B-016).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Writing—original draft preparation: X-QZ; Methodology: XZ; Investigation: YY, KD, HY; Writing—review and editing: LD, H-JZ; Supervision: LD, H-JZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This is retrospective research. The PUMCH Research Ethics Committee has confirmed that no ethical approval is required.
Consent to participate
Informed consent was obtained from all the study participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, XQ., Zhou, X., Yao, Y. et al. Acromegaly complicated with fulminant pituitary apoplexy: clinical characteristic analysis and review of literature. Endocrine 81, 160–167 (2023). https://doi.org/10.1007/s12020-023-03379-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03379-7