Abstract
Purpose
To describe the clinical features of a paediatric cohort affected by differentiated thyroid cancer (DTC) followed in a tertiary Department of Paediatric Endocrinology.
Methods
Clinical data of 41 patients affected by DTC in the 2000–2020 period were reviewed.
Results
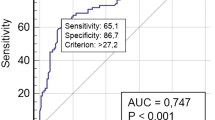
The main risk factor was autoimmune thyroiditis (39%). Cytological categories were TIR3b in 39%, TIR4 in 9.8%, TIR5 in 51.2%. After total thyroidectomy, radioiodine treatment was performed in 38 subjects (92.7%). ATA low-risk category was assigned in 11 (30.5%), intermediate-risk category in 15 (41.7%), and high-risk category in 10 patients (27.8%). Age at diagnosis was 15.1 ± 0.92 years in low-risk category, 14.7 ± 0.59 in intermediate-risk category, 11.7 ± 0.89 years in high-risk category (p = 0.01). TIR3b was manly observed in low-risk class (63.6%), while TIR5 was mainly reported in intermediate and high-risk class (60 and 80% respectively) (p = 0.04). Post-surgery stimulated thyroglobulin was increased in high-risk class (407.8 ± 307.1 ng/ml) [p = 0.04]. Tumour size was larger in high-risk category (42.6 ± 2.6 mm), than in low and intermediate-risk categories (19.4 ± 3.5 mm and 28.5 ± 3.9 mm, respectively) (p = 0.008). Patients in intermediate and high-risk categories displayed more tumour multifocality (60 and 90% respectively) (p < 0.005). Disease relapse was mainly observed in high risk category (40%, p = 0.04).
Conclusion
DTC in childhood is more aggressive than in adults, but the overall survival rate is excellent. The therapeutic approach is still heterogeneous, especially in low-risk category. Further studies are needed to standardise management and reduce disease persistence in childhood.
Similar content being viewed by others
References
M. Niedziela, Thyroid nodules. Best. Pr. Res Clin. Endocrinol. Metab. 28, 245–277 (2014). https://doi.org/10.1016/j.beem.2013.08.007
J.L. Sandy, A. Titmuss, S. Hameed, Y.H. Cho, G. Sandler, P. Benitez-Aguirre, Thyroid nodules in children and adolescents: Investigation and management. J. Paediatr. Child Health 58, 2163–2168 (2022). https://doi.org/10.1111/jpc.16257
M. Goldfarb, C. Dinauer, Differences in the management of thyroid nodules in children and adolescents as compared to adults. Curr. Opin. Endocrinol. Diabetes Obes. 29, 466–473 (2022). https://doi.org/10.1097/MED.0000000000000754
C.E. Cherella, T.E. Angell, D.M. Richman, M.C. Frates, C.B. Benson, F.D. Moore, J.A. Barletta, M. Hollowell, J.R. Smith, E.K. Alexander, E.S. Cibas, A.J. Wassner, Differences in thyroid nodule cytology and malignancy risk between children and adults. Thyroid 29, 1097–1104 (2019). https://doi.org/10.1089/thy.2018.0728
A. Corrias, A. Mussa, F. Baronio, T. Arrigo, M. Salerno, M. Segni, M.C. Vigone, R. Gastaldi, G. Zirilli, G. Tuli, L. Beccaria, L. Iughetti, S. Einaudi, G. Weber, F. De Luca, A. Cassio; Study Group for Thyroid Diseases of Italian Society for Pediatric Endocrinology and Diabetology (SIEDP/ISPED), Diagnostic features of thyroid nodules in pediatrics. Arch. Pediatr. Adolesc. Med 164, 714–719 (2010). https://doi.org/10.1001/archpediatrics.2010.114
A. Mussa, M.C. Salerno, G. Bona, M. Wasniewska, M. Segni, A. Cassio, M.C. Vigone, R. Gastaldi, L. Iughetti, A. Santanera, D. Capalbo, P. Matarazzo, F. De Luca, G. Weber, A. Corrias, Serum thyrotropin concentration in children with isolated thyroid nodules. J. Pediatr. 163, 1465–1470 (2013). https://doi.org/10.1016/j.jpeds.2013.07.003
R. Roy, G. Kouniavsky, E. Schneider, J.D. Allendorf, J.A. Chabot, P. Logerfo, A.P. Dackiw, P. Colombani, M.A. Zeiger, J.A. Lee, Predictive factors of malignancy in pediatric thyroid nodules. Surgery 150, 1228–1233 (2011). https://doi.org/10.1016/j.surg.2011.09.023
C.E. Cherella, H.A. Feldman, M. Hollowell, D.M. Richman, E.S. Cibas, J.R. Smith, T.E. Angell, Z. Wang, E.K. Alexander, A.J. Wassner, Natural history and outcomes of cytologically benign thyroid nodules in children. J. Clin. Endocrinol. Metab. 103, 3557–3565 (2018). https://doi.org/10.1210/jc.2018-00895
G. Tuli, J. Munarin, E. Agosto, P. Matarazzo, F. Quaglino, A. Mormile, L. de Sanctis, Predictive factors of malignancy in pediatric patients with thyroid nodules and performance of the Italian classification (SIAPEC 2014) in the outcome of the cytological FNA categories. Endocrine 74, 365–374 (2021). https://doi.org/10.1007/s12020-021-02784-0
G. Keefe, K. Culbreath, C.E. Cherella, J.R. Smith, B. Zendejas, R.C. Shamberger, D.M. Richman, M.L. Hollowell, B.P. Modi, A.J. Wassner, Autoimmune thyroiditis and risk of malignancy in children with thyroid nodules. Thyroid 32, 1109–1117 (2022). https://doi.org/10.1089/thy.2022.0241
C.A. Lebbink, T.P. Links, A. Czarniecka, R.P. Dias, R. Elisei, L. Izatt, H. Krude, K. Lorenz, M. Luster, K. Newbold, A. Piccardo, M. Sobrinho-Simões, T. Takano, A.S. Paul van Trotsenburg, F.A. Verburg, H.M. van Santen, 2022 European Thyroid Association Guidelines for the management of pediatric thyroid nodules and differentiated thyroid carcinoma. Eur. Thyroid J. 11, e220146 (2022). https://doi.org/10.1530/ETJ-22-0146
G.L. Francis, S.G. Waguespack, A.J. Bauer, P. Angelos, S. Benvenga, J.M. Cerutti, C.A. Dinauer, J. Hamilton, I.D. Hay, M. Luster, M.T. Parisi, M. Rachmiel, G.B. Thompson, S. Yamashita; American Thyroid Association Guidelines Task Force, Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 25, 716–759 (2015). https://doi.org/10.1089/thy.2014.0460
A. Cozzolino, T. Filardi, I. Simonelli, G. Grani, C. Virili, I. Stramazzo, M.G. Santaguida, P. Locantore, M. Maurici, D. Gianfrilli, A.M. Isidori, C. Durante, C. Pozza, Diagnostic accuracy of ultrasonographic features in detecting thyroid cancer in the transition age: a meta-analysis. Eur. Thyroid J. 11, e220039 (2022). https://doi.org/10.1530/ETJ-22-0039
G. Zirilli, L. Cannavò, F. Vermiglio, M.A. Violi, F. Luca, M. Wasniewska, Differentiated thyroid carcinoma presentation may be more aggressive in children and adolescents than in young adults. Ital. J. Pediatr. 44, 13 (2018). https://doi.org/10.1186/s13052-018-0455-3
A.J. Bauer, Pediatric thyroid cancer: genetics, therapeutics and outcome. Endocrinol. Metab. Clin. North Am. 49, 589–611 (2020). https://doi.org/10.1016/j.ecl.2020.08.001
M.E. Nikita, W. Jiang, S.M. Cheng, F.M. Hantash, M.J. McPhaul, R.O. Newbury, S.A. Phillips, R.E. Reitz, F.M. Waldman, R.S. Newfield, Mutational analysis in pediatric thyroid cancer and correlations with age, ethnicity, and clinical presentation. Thyroid 26, 227–234 (2016). https://doi.org/10.1089/thy.2015.0401
A.J. Bauer, Molecular genetics of thyroid cancer in children and adolescents. Endocrinol. Metab. Clin. North Am. 46, 389–403 (2017). https://doi.org/10.1016/j.ecl.2017.01.014
A.S. Remiker, J. Chuang, S. Corathers, M.M. Rutter, M.J. Rutter, C.M. Myer IV, M.J. Gelfand, A.T. Trout, J.I. Geller, Differentiated thyroid cancer in the pediatric/adolescent population: evolution of treatment. J. Pediatr. Hematol. Oncol. 41, 532–536 (2019). https://doi.org/10.1097/MPH.0000000000001493
A.B. Zanella, R.S. Scheffel, L. Weinert, J.M. Dora, A.L. Maia, New insights into the management of differentiated thyroid carcinoma in children and adolescents (Review). Int J. Oncol. 58, 13 (2021). https://doi.org/10.3892/ijo.2021.5193
C.S. O’Gorman, J. Hamilton, M. Rachmiel, A. Gupta, B.Y. Ngan, D. Daneman, Thyroid cancer in childhood: a retrospective review of childhood course. Thyroid 20, 375–380 (2010). https://doi.org/10.1089/thy.2009.0386
P. Papendieck, L. Gruñeiro-Papendieck, M. Venara, O. Acha, S. Maglio, I. Bergadá, A. Chiesa, Differentiated thyroid carcinoma: presentation and follow-up in children and adolescents. J. Pediatr. Endocrinol. Metab. 24, 743–748 (2011). https://doi.org/10.1515/jpem.2011.241
L.B. Vergamini, A.L. Frazier, F.L. Abrantes, K.B. Ribeiro, C. Rodriguez-Galindo, Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J. Pediatr. 164, 1481–1485 (2014). https://doi.org/10.1016/j.jpeds.2014.01.059
K. Sugino, M. Nagahama, W. Kitagawa, H. Shibuya, K. Ohkuwa, T. Uruno, A. Suzuki, J. Akaishi, C. Masaki, K. Matsuzu, K. Ito, Papillary thyroid carcinoma in children and adolescents: long-term follow-up and clinical characteristics. World J. Surg. 39, 2259–2265 (2015). https://doi.org/10.1007/s00268-015-3042-4
S. Golpanian, J. Tashiro, J.E. Sola, C. Allen, J.I. Lew, A.R. Hogan, H.L. Neville, E.A. Perez, Surgically treated pediatric nonpapillary thyroid carcinoma. Eur. J. Pediatr. Surg. 26, 524–532 (2016). https://doi.org/10.1055/s-0035-1569150
M.S. Klein Hesselink, M. Nies, G. Bocca, A.H. Brouwers, J.G. Burgerhof, E.W. van Dam, B. Havekes, M.M. van den Heuvel-Eibrink, E.P. Corssmit, L.C. Kremer, R.T. Netea-Maier, H.J. van der Pal, R.P. Peeters, K.W. Schmid, J.W. Smit, G.R. Williams, J.T. Plukker, C.M. Ronckers, H.M. van Santen, W.J. Tissing, T.P. Links, Pediatric differentiated thyroid carcinoma in the Netherlands: a nationwide follow-up study. J. Clin. Endocrinol. Metab. 101, 2031–2039 (2016). https://doi.org/10.1210/jc.2015-3290
I.D. Hay, T.R. Johnson, S. Kaggal, M.S. Reinalda, N.M. Iniguez-Ariza, C.S. Grant, S.T. Pittock, G.B. Thompson, Papillary thyroid carcinoma (PTC) in children and adults: comparison of initial presentation and long-term postoperative outcome in 4432 patients consecutively treated at the mayo clinic during eight decades (1936-2015). World J. Surg. 42, 329–342 (2018). https://doi.org/10.1007/s00268-017-4279-x
A. Zbitou, E. Desandes, S. Guissou, C. Mallebranche, B. Lacour, Thyroid cancers in children and adolescents in France: Incidence, survival and clinical management over the 2000-2018 period. Int J. Pediatr. Otorhinolaryngol. 162, 111325 (2022). https://doi.org/10.1016/j.ijporl.2022.111325
K.A. Lee, M.T.A. Sharabiani, D. Tumino, J. Wadsley, V. Gill, G. Gerrard, R. Sindhu, M.N. Gaze, L. Moss, K. Newbold, Differentiated thyroid cancer in children: a UK multicentre review and review of the literature. Clin. Oncol. 31, 385–390 (2019). https://doi.org/10.1016/j.clon.2019.02.005
M. Negre Busó, A. García Burillo, M. Simó Perdigó, P. Galofré Mora, M. Boronat de Ferrater, G. Cuberas Borrós, C. Sábado Álvarez, J. Castell Conesa, Long-term follow-up of differentiated thyroid carcinoma in children and adolescents. J. Pediatr. Endocrinol. Metab. 33, 1431–1441 (2020). https://doi.org/10.1515/jpem-2020-0194
G.L. Banik, M.L. Shindo, K.L. Kraimer, K.L. Manzione, A. Reddy, K. Kazahaya, A.J. Bauer, J.C. Rastatter, M.E. Zafereo, S.G. Waguespack, D.C. Chelius Jr, L. Quintanilla-Dieck, Prevalence and risk factors for multifocality in pediatric thyroid cancer. JAMA Otolaryngol. Head. Neck Surg. 147, 1100–1106 (2021). https://doi.org/10.1001/jamaoto.2021.3077
C.E. Cherella, D.M. Richman, E. Liu, M.C. Frates, B.P. Modi, B. Zendejas, J.R. Smith, J.A. Barletta, M.L. Hollowell, A.J. Wassner, Predictors of bilateral disease in pediatric differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 106, 4242 (2021). https://doi.org/10.1210/clinem/dgab210
C.S. Bal, A. Garg, S. Chopra, S. Ballal, R. Soundararajan, Prognostic factors in pediatric differentiated thyroid cancer patients with pulmonary metastases. J. Pediatr. Endocrinol. Metab. 28, 745–751 (2015). https://doi.org/10.1515/jpem-2014-0247
K. Sugino, M. Nagahama, W. Kitagawa, K. Ohkuwa, T. Uruno, K. Matsuzu, A. Suzuki, C. Tomoda, K.Y. Hames, J. Akaishi, C. Masaki, K. Ito, Distant metastasis in pediatric and adolescent differentiated thyroid cancer: clinical outcomes and risk factor analyses. J. Clin. Endocrinol. Metab. 105, dgaa545 (2020). https://doi.org/10.1210/clinem/dgaa545
O. Karapanou, M. Tzanela, P. Rondogianni, C. Dacou-Voutetakis, D. Chiotis, B. Vlassopoulou, D. Vassiliadi, C. Kanaka-Gantenbein, S. Tsagarakis, Long-term outcome of differentiated thyroid cancer in children and young adults: risk stratification by ATA criteria and assessment of pre-ablation stimulated thyroglobulin as predictors of disease persistence. Endocrine 70, 566–574 (2020). https://doi.org/10.1007/s12020-020-02378-2
A. Zanella, R.S. Scheffel, M.W. Pasa, J.M. Dora, A.L. Maia, Role of postoperative stimulated thyroglobulin as prognostic factor for differentiated thyroid cancer in children and adolescents. Thyroid 27, 787–792 (2017). https://doi.org/10.1089/thy.2016.0559
N.K. Jain, S. Mostoufi-Moab, C.P. Hawkes, N.D. Nelson, L.F. Surrey, Z.S. Jones, N.S. Adzick, K. Kazahaya, A.J. Bauer, Extrathyroidal extension is an important predictor of regional lymph node metastasis in pediatric differentiated thyroid cancer. Thyroid 30, 1037–1043 (2020). https://doi.org/10.1089/thy.2019.0229
E.J. Propst, J.D. Wasserman, J. Gorodensky, B.Y. Ngan, N.E. Wolter, Patterns and predictors of metastatic spread to the neck in pediatric thyroid carcinoma. Laryngoscope 131, 1002 (2021). https://doi.org/10.1002/lary.28937
J.C. Rubinstein, K. Herrick-Reynolds, C. Dinauer, R. Morotti, D. Solomon, G.G. Callender, E.R. Christison-Lagay, Recurrence and complications in pediatric and adolescent papillary thyroid cancer in a high-volume practice. J. Surg. Res 249, 58–66 (2020). https://doi.org/10.1016/j.jss.2019.12.002
E. Christison-Lagay, R.M. Baertschiger, Management of differentiated thyroid carcinoma in pediatric patients. Surg. Oncol. Clin. N. Am. 30, 235–251 (2021). https://doi.org/10.1016/j.soc.2020.11.013
Y.A. Lee, H.W. Jung, H.Y. Kim, H. Choi, H.Y. Kim, J.H. Hah, D.J. Park, J.K. Chung, S.W. Yang, C.H. Shin, Y.J. Park, Pediatric patients with multifocal papillary thyroid cancer have higher recurrence rates than adult patients: a retrospective analysis of a large pediatric thyroid cancer cohort over 33 years. J. Clin. Endocrinol. Metab. 100, 1619–1629 (2015). https://doi.org/10.1210/jc.2014-3647
S.R. Howard, S. Freeston, B. Harrison, L. Izatt, S. Natu, K. Newbold, S. Pomplun, H.A. Spoudeas, S. Wilne, T.R. Kurzawinski, M.N. Gaze, Paediatric differentiated thyroid carcinoma: a UK National Clinical Practice Consensus Guideline. Endocr. Relat. Cancer 29, 1 (2022). https://doi.org/10.1530/ERC-22-0035
W.P. Kluijfhout, J.D. Pasternak, D. van der Kaay, M.R. Vriens, E.J. Propst, J.D. Wasserman, Is it time to reconsider lobectomy in low-risk paediatric thyroid cancer? Clin. Endocrinol. 86, 591–596 (2017). https://doi.org/10.1111/cen.13287
C.K. Sudoko, C.M. Jenks, A.J. Bauer, A. Isaza, S. Mostoufi-Moab, L.F. Surrey, T.R. Bhatti, A. Franco, N.S. Adzick, K. Kazahaya, Thyroid lobectomy for T1 papillary thyroid carcinoma in pediatric patients. JAMA Otolaryngol. Head. Neck Surg. 147, 943–950 (2021). https://doi.org/10.1001/jamaoto.2021.2359
L. Lazar, Y. Lebenthal, K. Segal, A. Steinmetz, Y. Strenov, M. Cohen, I. Yaniv, M. Yackobovitch-Gavan, M. Phillip, Pediatric thyroid cancer: postoperative classifications and response to initial therapy as prognostic factors. J. Clin. Endocrinol. Metab. 101, 1970–1979 (2016). https://doi.org/10.1210/jc.2015-3960
G. Radetti, S. Loche, V. D’Antonio, M. Salerno, C. Guzzetti, T. Aversa, A. Cassio, M. Cappa, R. Gastaldi, F. Deluca, M.C. Vigone, G.M. Tronconi, A. Corrias, Influence of Hashimoto Thyroiditis on the development of thyroid nodules and cancer in children and adolescents. J. Endocr. Soc. 3, 607–616 (2019). https://doi.org/10.1210/js.2018-00287
W.L. Ho, M.R. Zacharin, Thyroid carcinoma in children, adolescents and adults, both spontaneous and after childhood radiation exposure. Eur. J. Pediatr. 175, 677–683 (2016). https://doi.org/10.1007/s00431-016-2692-z
C.A. Lebbink, S.G. Waguespack, H.M. van Santen, Thyroid dysfunction and thyroid cancer in childhood cancer survivors: prevalence, surveillance and management. Front. Horm. Res. 54, 140–153 (2021). https://doi.org/10.1159/000513805
H.M. van Santen, E.K. Alexander, S.A. Rivkees, E. Frey, S.C. Clement, M.P. Dierselhuis, C.A. Lebbink, T.P. Links, K. Lorenz, R.P. Peeters, C. Reiners, M.R. Vriens, P. Nathan, A.B. Schneider, F. Verburg, Clinical considerations for the treatment of secondary differentiated thyroid carcinoma in childhood cancer survivors. Eur. J. Endocrinol. 183, 1 (2020). https://doi.org/10.1530/EJE-20-0237
S.C. Clement, C.A. Lebbink, M.S. Klein Hesselink, J.C. Teepen, T.P. Links, C.M. Ronckers, H.M. van Santen, Presentation and outcome of subsequent thyroid cancer among childhood cancer survivors compared to sporadic thyroid cancer: a matched national study. Eur. J. Endocrinol. 183, 169–180 (2020). https://doi.org/10.1530/EJE-20-0153
W. Chemaitilly, C.A. Sklar, Childhood cancer treatments and associated endocrine late effects: a concise guide for the pediatric endocrinologist. Horm. Res Paediatr. 91, 74–82 (2019). https://doi.org/10.1159/000493943
J.D. Pole, A.M. Zuk, J.D. Wasserman, Diagnostic and treatment patterns among children, adolescents, and young adults with thyroid cancer in Ontario: 1992-2010. Thyroid 27, 1025–1033 (2017). https://doi.org/10.1089/thy.2016.0629
D. Albano, F. Bertagna, M.B. Panarotto, R. Giubbini Early and late adverse effects of radioiodine for pediatric differentiated thyroid cancer. Pediatr. Blood Cancer. 64 (2017). https://doi.org/10.1002/pbc.26595
E. Pasqual, S. Schonfeld, L.M. Morton, D. Villoing, C. Lee, A. Berrington de Gonzalez, C.M. Kitahara, Association between radioactive iodine treatment for pediatric and young adulthood differentiated thyroid cancer and risk of second primary malignancies. J. Clin. Oncol. 40, 1439–1449 (2022). https://doi.org/10.1200/JCO.21.01841
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design and conception. Material preparation, data collection and analysis were performed by A.M., J.M. and G.T. The clinical management of the enroled subjects was managed by A.C., P.M., G.T., J.M., F.Q. and L.D. Sanctis. The first draft of the manuscript was written by G.T. and J.M. and all authors have commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to publish
Consent to publish has been received from all partecipants.
Consent to partecipate
Written parental consent was obtained.
Ethics approval
This study was conducted in line with the principles of the Declaration of Helsinki. The approval was granted by the Ethics Committee of the City of Health and Science University Hospital of Turin (01/10/2021, 13942/A1).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tuli, G., Munarin, J., Matarazzo, P. et al. Clinical features of thyroid cancer in paediatric age. Experience of a tertiary centre in the 2000–2020 period. Endocrine 81, 322–329 (2023). https://doi.org/10.1007/s12020-023-03366-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03366-y