Abstract
Introduction
Gestational diabetes (GDM) and pre-eclampsia (PE) represents the unrecognized risk factors for reduced bone content in neonates. The present study is planned to explore the components of vitamin D metabolism and calcium transport in placenta of GDM and PE cases and its effect on the neonatal bone mass determination using bone densitometry system.
Methods
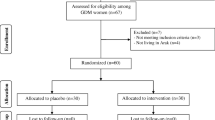
We have collected serum and placenta tissues from GDM (n = 20), PE (n = 20), and healthy pregnancies (n = 20). In the present study, we found mRNA expression of oxidative stress markers, vitamin D metabolic components and calcium channels, calcium channel binding proteins, plasma membrane calcium ATPase, ATP synthase and Ca2+ release genes; Ryanodine receptors genes were assessed by quantitative real-time PCR (qRT-PCR) in placental tissue of GDM, PE, and healthy pregnancies.
Results
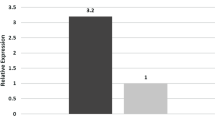
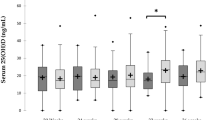
We observed high level of oxidative stress in both GDM and PE placenta compared to normal pregnancies. CYP2R1 and VDR mRNA expression was significantly downregulated and upregulation of CYP27B1 and CYP24A1 in GDM and PE compared with healthy cases. Similarly, calcium transporters were downregulated in GDM and PE placental tissues. In addition, CYP24A1, VDR, CaBP28K, TRPV5 and PMCA3 mRNA expression were correlated with BMC of neonates.
Discussion
Oxidative stress is probably relevant to disrupted vitamin D homeostasis and calcium transport in the placenta of GDM and PE cases. The altered regulatory mechanism of CYP24A1 and VDR could indicates more pronounced serum 25(OH)D reduction. Additionally, reduced BMC in the neonates of these cases might be as consequences of modified CYP24A1, VDR, CaBP28K, TRPV5 and PMCA3 mRNA expression.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
P. Murthi, H.E. Yong, T.P. Ngyuen et al. Role of the placental vitamin D receptor in modulating feto placental growth in fetal growth restriction and preeclampsia-affected pregnancies. Front. Physiol. 18(7), 43 (2016)
F. Haidari, M.T. Jalali, N. Shahbazian, M.H. Haghighizadeh, E. Azadegan, Comparison of serum levels of vitamin D and inflammatory markers between women with gestational diabetes mellitus and healthy pregnant control. J. Fam. Reprod. Health 10, 1–8 (2016)
G.J. Cho, S.C. Hong, M.J. Oh, H.J. Kim, Vitamin D deficiency in gestational diabetes mellitus and the role of the placenta. Am. J. Obstet. Gynecol. 209, 560.e1–8 (2013)
C. Lechtermann, B.P. Hauffa, R. Herrmann et al. Maternal vitamin D status in preeclampsia: seasonal changes are not influenced by placental gene expression of vitamin D metabolizing enzymes. PLoS ONE 22(9), e105558 (2014)
L.M. Bodnar, J.M. Catov, H.N. Simhan, M.F. Holick, R.W. Powers, J.M. Roberts, Maternal vitamin D deficiency increases the risk of preeclampsia. J. Clin. Endocrinol. Metab. 92, 3517–3522 (2007)
N. Yates, R.C. Crew, C.S. Wyrwoll, Vitamin D deficiency and impaired placental function: potential regulation by glucocorticoids? Reproduction 153(5), R163–R171 (2017)
R.J. Moon, N.C. Harvey, C. Cooper, Influence of maternal vitamin D status on obstetric outcomes and the foetal skeleton. Eur. J. Endocrinol. 173, R69–R83 (2015)
R. Adela, R.M. Borkar, M.M. Bhandi, G. Vishwakarma, R. Srinivas, P.N.C. Reddy, S.K. Banerjee, Lower vitamin D metabolites levels in Indian diabetic patients were associated with coronary artery diseases. Sci. Rep. 6, 37593 (2016)
B. Novakovic, M. Sibson, H.K. Ng, U. Manuelpillai, V. Rakyan, T. Down et al. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J. Biol. Chem. 284, 14838–14848 (2009)
N.J. Oviedo, G. Benaim, V. Cervino et al. The plasma membrane Ca2-ATPase protein from red blood cells is not modified in preeclampsia. Biochim. Biophys. Acta 1762, 381–385 (2006)
R. Martin, N.C. Harvey, S.R. Crozier et al. Placental calcium transporter (PMCA3) gene expression predicts intrauterine bone mineral accrual. Bone 40, 1203–1208 (2007)
B. Ashley, C. Simner, A. Manousopoulou, C. Jenkinson, Placental uptake and metabolism of 25(OH)vitamin D determine its activity within the fetoplacental unit. eLife 11, e71094 (2022)
L. Belkacemi, U. Zuegel, A. Steinmeyer, J.P. Dion, J. Lafond, Calbindin-D28k (CaBP28k) identification and regulation by 1,25-dihydroxyvitamin D3 in human choriocarcinoma cell line JEG-3. Mol. Cell Endocrinol. 236, 31–41 (2005)
International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycaemia in pregnancy. Diabetes Care 33, 676–682 (2010)
M.H. Schoots, S.J. Gordijn, S.A. Scherjon, H. van Goor, J.L. Hillebrands, Oxidative stress in placental pathology. Placenta 69, 153–161 (2018)
R. Adela, R.M. Borkar, N. Mishra et al. Lower serum vitamin D metabolite levels in relation to circulating cytokines/chemokines and metabolic hormones in pregnant women with hypertensive disorders. Front Immunol. 8, 273 (2017)
R. Ma, Y. Gu, S. Zhao, J. Sun, L.J. Groome, Y. Wang, Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 303, E928–E935 (2012)
S. Ching, S. Kashinkunti, M.D. Niehaus, G.M. Zinser, Mammary adipocytes bioactivate 25-hydroxyvitamin D (3) and signal via vitamin D (3) receptor, modulating mammary epithelial cell growth. J. Cell Biochem 112, 3393–3405 (2011)
S. Varshney, S.K. Bhadada, N. Sachdeva et al. Methylation status of the CpG islands in vitamin D and calcium-sensing receptor gene promoters does not explain the reduced gene expressions in parathyroid adenomas. J. Clin. Endocrinol. Metab. 98, E1631–E1635 (2013)
B.E. Young, E.M. Cooper, A.W. McIntyre et al. Placental vitamin D receptor (VDR) expression is related to neonatal vitamin D status, placental calcium transfer, and fetal bone length in pregnant adolescents. FASEB J. 28, 2029–2037 (2014)
M.B. Meyer, M. Watanuki, S. Kim, N.K. Shevde, J.W. Pike, The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol. Endocrinol. 20, 1447–1461 (2006)
L. Belkacemi, U. Zuegel, A. Steinmeyer, J.P. Dion, J. Lafond, Calbindin-D28k (CaBP28k) identification and regulation by 1,25-dihydroxyvitamin D3 in human choriocarcinoma cell line JEG-3. Mol. Cell. Endocrinol. 236, 31–41 (2005)
A. Halhali, A.G. Figueras, L. Diaz et al. Effects of calcitriol on calbindins gene expression and lipid peroxidation in human placenta. J. Steroid Biochem Mol. Biol. 121, 448–451 (2010)
S. Haché, L. Takser, F. LeBellego et al. Alteration of calcium homeostasis in primary preeclamptic syncytiotrophoblasts: effect on calcium exchange in placenta. J. Cell Mol. Med 15, 654–667 (2011)
J.B. Peng, E.M. Brown, M.A. Hediger, Epithelial Ca2+ entry channels: transcellular Ca2+ transport and beyond. J. Physiol. 551, 729–740 (2003)
J.G. Hoenderop, J.P. van Leeuwen, B.C. van der Eerden et al. Renal Ca2 wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Investig. 112, 1906–1914 (2003)
Y. Suzuki, C.S. Kovacs, H. Takanaga et al. Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J. Bone Min. Res. 23, 1249–1256 (2008)
T.T. Lambers, F. Mahieu, E. Oancea et al. Calbindin-D28K dynamically controls TRPV5-mediated Ca2_ transport. EMBO J. 25, 2978–2988 (2006)
J.G. Hoenderop, J.P. van Leeuwen, B.C. van der Eerden et al. Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J. Clin. Investig. 112, 1906–1914 (2003)
J. Lafond, L. Simoneau, Calcium homeostasis in human placenta: role of calcium handling proteins. Int Rev. Cytol. 250, 109–174 (2006)
S.R. Hansson, Y. Chen, J. Brodszki et al. Gene expression profiling of human placentas from preeclamptic and normotensive pregnancies. Mol. Hum. Reprod. 12, 169–179 (2006)
T. Nabekura, M. Tomohiro, Y. Ito et al. Changes in plasma membrane Ca2-ATPase expression and ATP content in lenses of hereditary cataract UPL rats. Toxicology 197, 177–183 (2004)
T. Cindrova-Davies, Gabor Than Award Lecture 2008: pre-eclampsia–from placental oxidative stress to maternal endothelial dysfunction. Placenta 30, S55–S65 (2009)
J.D. Glazier, D.E. Atkinson, K.L. Thornburg et al. Gestational changes in Ca2+ transport across rat placenta and mRNA for calbindin9K and Ca(2+)-ATPase. Am. J. Physiol. 263, R930–R935 (1992)
E. Herrera, E. Amusquivar, Lipid metabolism in the fetus and the newborn. Diabetes/Metab. Res. Rev. 16(3), 202–210 (2000)
M. Takahashi, S. Makino, K. Oguma, H. Imai et al. Fetal growth restriction as the initial finding of preeclampsia is a clinical predictor of maternal and neonatal prognoses: a single-center retrospective study. BMC Pregnancy Childbirth 21(1), 1–8 (2021)
M.J. Berridge, M.D. Bootman, H.L. Roderick, Calcium signaling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 (2003)
Acknowledgements
We acknowledge Ms. Tarang Gupta, Senior research fellow, Department of Endocrinology & Metabolism for her support in sample collection. S.V. is thankful to University Grant Commission (UGC) for providing postdoctoral fellowship for women (File No.F.15-1/2016-17/PDFWM-2015-17-UTT-37224 (SA-II)). S.V. also thanks full to Indian Society for Bone & Mineral Research (ISBMR) for providing financial support. R.Adela is thankful to Department of Science and Technology for providing the National Postdoctoral Research Fellowship (PDF/2016/000779).
Author contributions
S.V. and R.Adela contributed to study designing, sample collection, gene expression analysis, clinical data collection, data analysis, interpretation, and manuscript writing. G.K., V.K., and R.Kumari contributed to the study designing, interpretation, gynecological review. R.D. contributed to study designing, molecular data interpretation, and review. R.Agarwal contributed to the study designing, interpretation, and neonatal opinion. R.Khadgawat contributed to the study designing, interpretation, endocrine review. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Funding
S.V. is thankful to University Grant Commission (UGC) for providing postdoctroal fellowship for women (File No. F.15-1/2016-17/PDFWM-2015-17-UTT-37224 (SA-II)). R.Adela is thankful to Department of Science and Technology for providing National Postdoctoral Research Fellowship (PDF/2016/000779). S.V. received financial support from Indian Society for Bone and Mineral Research (ISBMR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was reviewed and approved by the institutional ethics committee of the All India Institute of Medical Sciences-New Delhi (IEC/414/8/2016). The clinical study was conducted in accordance with principles outlined in the Declaration of Helsinki and institution and ethical standards. The individuals supplied written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Varshney, S., Adela, R., Kachhawa, G. et al. Disrupted placental vitamin D metabolism and calcium signaling in gestational diabetes and pre-eclampsia patients. Endocrine 80, 191–200 (2023). https://doi.org/10.1007/s12020-022-03272-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03272-9