Abstract
Background—Purpose
Randomized controlled trials (RCTs) are the cornerstone of evidence-based medicine, yet their quality is often suboptimal. The Consolidated Standards of Reporting Trials (CONSORT) statement is a list of advice to upgrade the quality of RCTs. The aim of this study was the assessment of the quality of RCTs for vitamin D supplements in thyroid autoimmunity according to the revised CONSORT 2010 checklist.
Methods
Databases were searched for RCTs involving patients with autoimmune thyroid disorders (AITDs) who received vitamin D supplements published from 2011 to 2021. A list of 37-items was used and adherence ≥75% was considered of optimal quality. The primary outcome was the mean CONSORT adherence of studies. Secondary outcomes were the estimation of compliance per CONSORT item and the examination for possible determinants of the reporting quality.
Results
Thirteen eligible trials were finally included. The mean compliance was 61.15% ± 14.86%. Only threeof the studies (23%) achieved a good reporting quality (≥75%), while ten (77%) were presented with inadequate reporting (<75%). Randomization and blinding were mainly poorly reported. Impact Factor (IF) of journal was associated with the reporting quality in the univariate analysis [p = 0.033, OR = 1.65, 95%CI = (1316, 1773)]. Sample size (p = 0.067), number of authors (p = 0.118) and number of citations (p = 0.125) were marginally not significant. None of the factors showed significant results in multivariate analysis. Reporting quality and IF were strongly positively correlated [Pearson’s r = 0.740, p = 0.04].
Conclusion
This study shows that mean CONSORT adherence of RCTs for Vitamin D supplementation in AITDs is moderate, reflecting that study quality and transparency could be improved with better adherence to CONSORT rules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of Autoimmune thyroid diseases (AITDs) is about five percent which rendersthem the most common amongautoimmune disorders with a continuing rise in incidence. Thefemale populationis at a greater riskof developing thyroid autoimmunity than men [1]. The most common AITDs are Hashimoto thyroiditis (HT), Graves’ disease (GD) among the general population and post-partum thyroiditis (PPT) in pregnant women.AITDare caused by multiple factors, involving both environmentaland genetic factors [2,3,4].
Vitamin D is a secosteroidal hormone precursor and has been identified as a key hormone inthe musculoskeletal, nervous system and insulin sensitivity [5,6,7]. Several studies have reported a low vitamin D status in AITD, indicating an association between vitamin D deficiency and thyroid autoimmunity [8,9,10,11,12,13]. On the other hand, a small number of studies,showed no significant association between AITDs and vitamin D deficiency [14,15,16,17]. These pieces of evidence led several researchers to examine the effectiveness of vitamin D supplementation in the prevention/treatment of this group of conditions [18, 19]. The results are conflicting, so the potential of vitamin D in thyroid diseases treatmentneeds to be clarified.
Double-blind RCTs are considered to be the highest ranked mean of evidence-based medicine and their results are crucial in the formulation of the therapeutic guidelines [20]. RCTs represent better the whole strategy and philosophy of the research [21].
Readers have access to a plethora of articles, so there is a need for a tool to assess the guidance of RCTs [22].
In 1996, an international group of experts created the CONSORT (Consolidated Standards of Reporting Trials) Statement [23]. Two revisions followed in 2001 and 2010with detailed explanation and elaboration documents [24, 25]. This statement is an evidence-based set of advice, including a checklist of 37 items and a flow diagram whose reporting ensures the avoidance of failing to include important information [25]. For that reason, an increasing number of journals endorse compliance with the CONSORT statement to improve reporting standards [26].
The quality of RCTshas beeninvestigated in a variety of specialties [27,28,29,30,31]. Our team, in a previous study concerning anticoagulant versus antiplatelet medication for venous thromboembolism prophylaxis, the average CONSORT compliance score was found to be 59.69% (38–83%). Only one RCT achievedmore than 75% of the CONSORT items (83%) [32].
To our knowledge, no published study has evaluated the quality of RCTs for vitamin D supplement in thyroidautoimmunity based on the CONSORT statement. The most recent study published in December 2021 was a meta-analysis focusing on cases of Hashimoto disease and the evaluation was conducted using the Cochrane CollaborationRisk of Bias tool Statistical analysis [19].
The purpose of this study is to evaluate the reporting quality of RCTsfor vitamin D supplementation in autoimmune thyroid disorders according to Consortstatement covering a period from January 2011, onwards following the release of the updated CONSORT 2010 guidelines in March 2010, until December 31st, 2021.
Methods
Data sources and search strategies
An electronic structured literature search was organized using the following databases MEDLINE/PubMed, Cochrane library and Google Scholar. We attempted to identify relevant RCTs published within the time period from January 2011 onwards following the release of the updated CONSORT 2010 guidelines in March 2010, until December 31st, 2021.
The implemented combination of the following terms is reproduced:
(((((“Vitamin D”[Mesh] OR “Ergocalciferols”[Mesh] OR “Vitamin D Response Element”[Mesh] OR “Vitamin D-Binding Protein”[Mesh] OR “Vitamin D Deficiency”[Mesh] OR “Receptors, Calcitriol”[Mesh] OR “Vitamin D3 24-Hydroxylase”[Mesh] OR “vitamin D-binding protein-macrophage activating factor” [Supplementary Concept] OR “Cholecalciferol”[Mesh] OR “MED4 protein, human” [Supplementary Concept] OR “vitamin D binding protein 2, primate” [Supplementary Concept] OR “vitamin D binding protein 1, primate” [Supplementary Concept] OR “vitamin D response element-binding protein 2” [Supplementary Concept] OR “vitamin D 1-alpha hydroxylase” [Supplementary Concept] OR “vitamin D3 glucosiduronate” [Supplementary Concept]) OR (“Calcitriol”[Mesh] OR “25-O-ethyl-calcitriol” [Supplementary Concept] OR “22-dehydro-1,25-dihydroxy-24-dihomovitamin D3” [Supplementary Concept] OR “24,24-difluoro-1,25-dihydroxy-26,27-dimethylvitamin D3” [Supplementary Concept] OR “1,25-dihydroxyvitamin D3-23,26-lactol” [Supplementary Concept] OR “Vitamin D supplementation”)) AND (“Hashimoto Disease”[Mesh] OR “Hypothyroidism, Autoimmune” [Supplementary Concept])) OR (“Thyroiditis”[Mesh] OR “Postpartum Thyroiditis”[Mesh] OR “Thyroiditis, Autoimmune”[Mesh] OR “Thyroiditis, Chronic” [Supplementary Concept])) OR “Hypothyroidism”[Mesh]) OR (“anti-thyroid autoantibodies” [Supplementary Concept] OR “Autoantibodies”[Mesh] OR Graves’ disease OR Hyperthyroidism OR postpartum thyroiditis).
In order to restrict the search in PubMed, the “Randomized Controlled Trial”filter for study type, the “English” filter for language and lastly the “Humans”species filterwere used.
Eligibility of studies
Inclusioncriteria:
-
Published from January 1st 2011 until December 31st, 2021
-
Parallel groupRCTs
-
One group was randomized to receive calcitriol or other Vitamin D analogs
-
They recruit patients with autoimmune thyroid disease
Exclusion criteria:
-
Non-randomized studies
-
Reviews
-
Pilot studies
-
Non-human studies
-
Studies with crossover design
-
Economic analyses
-
Small pilot studies
-
Study protocols
-
Articles not in English
Reporting assessment tool
The revised CONSORT checklist was used, which includes a 37-item questionnaire [25]. The CONSORT elaboration and explanationstatementguided the process [33]. CONSORT offersrecommendations for eachpart of an RCT, such as title, introduction, methods, results, discussion or other information, coveringall aspects of an optimal clinical trial [34].
The immediate period (until December 31st2010) following the publication of the latest revision of CONSORT statement (Mar 2010) was not included in the assessment. This decision was made to provide authors with enough time to abide by the revisedrecommendations.
Methodological evaluation
During the evaluation process, the selected articles were reviewed one by one according to the revised CONSORT version of 2010.Each item was appraised one of the following scores: ‘yes’ 1 point when adequately reported, ‘no’ or ‘unclear’ 0 points when inadequately reported or absent. When an item was reported in a different section of the trial, it was considered as a positive response.Regarding items on the CONSORT check- list with statements such as “When applicable” (7b), “If done” (11a)or “If relevant” (11b) they were checked as “non-applicable” if the answer was definite yes or no; then the answer of these items wasanalyzed accordingly.This resulted in a score range from 0 to 37.
Additional information included publication year,journal ranking [5-year Impact Factor (IF)published in 2020 by Clarivate Analytics via Journal Citation Reports], reporting of funding sources, number of authors, continent of first author, sample size, number of citations.
Outcome measures and Statistical analysis
The period from January 2011until December 31st, 2021 was assessed.It was decided that the remaining part of 2020 would not be evaluated in order to provide a sufficient time for authors to conform with the newest recommendations.The primary outcome measure was the mean CONSORT adherence of the included RCTs.Compliance above 75% with the CONSORT items was regarded as cut-off [31, 32]. We investigated the adherence of each itemseparately and the existence of possible determinant factors were also investigated.
All parameters were analyzed as categorical variables:IF (<2.86, ≥2.86 based on the median of our sample), sample size (≥82, <82 based on the median of our sample), citations (≥ 5, <5 based on the median of our sample), number of authors(≥7, <7 based on the median of our sample), funding source(yes/no), Covid-19 pandemic(earlier/in the course of). Pearson’s chi squared test (or Fisher’s exact test) was used for univariate analysis.A relaxed p-value of 0.20 was established arbitrary as a cut-off value in order to enter the binary logistic regression. A strict P value of 0.05 was set to be important for the multivariate analysis. Odds ratios (ORs), 95% confidence intervals (95% Cls) and P value are presented. An additional analysis was performed in order to examine a possible linear correlation between IF and reporting quality.SPSS v.26 package was used for statistical analysis.
Ethical view
No approval from any Ethical committee was sought, since this study analyzed existing data from publicly available sources.
Results
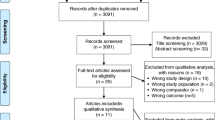
Initially, 8196 studies were obtained through the selected databases(Pubmed, Cochrane library and Google scholar). After removal of duplicated items, 6467 records were remained. Following evaluation of title and abstracts, 20 potentially eligible articles were identified. Finally, the full-text of these studies were examined and 13 studies were included in further assessment. Fig. 1 describes the five steps of the search strategy in a PRISMA flow diagram.
CONSORTadherence
The mean compliance to the CONSORT statement for RCTs was calculated at 61.15% with SD = 14.86% (Median = 62%, minimum & maximum adherence were 38% and 86% respectively). Among the studies, only 3 (23%) achieved a good reporting quality (≥75% of the items), while 10 (77%) presented with inadequate reporting (< 75% of the items). The mean proportion of adherence to the CONSORT statement for each study are presented in Table 1 and Fig. 2.
Adherence per CONSORT item was estimated (Table 2, Fig. 3). Specially, 5 of the 37 items of the checklist (13.5%) were reported in all (100%) of the articles and only 16 of the 37 items of the checklist (43.2%) were reported by 75% or more of the studies. Among methodological items, randomization process (items 8a and 8b) and blinding (items 10 and 11a) were mainly inadequately reported. In contrast, a structured abstract (item 1b) was reported adequately (77%) among the studies and is considered of crucial importance, taking into account that most readers base their decision to acquire or not a full text on its abstract.
Determinants of reporting quality
According to univariate analysis high IF of journal was the only with superior statistical significance (p < 0.05). Large sample size, great number of authors, existence of funding source was all associated with an adequate p value (p < 0.20) in order to enter binary logistic regression. Results are summarized at Table 3.
The four predictors of the univariate analysis were entered into a multivariable model. None of these was associated significantly with adequate reporting. Particularly, the journal impact factor (p = 0.150) failed to demonstrate significant effect, whereas the effect of number of citations (p = 0.650), sample size (p = 0.161) and number of authors (p = 0.892) persisted inadequately. Results of binary logistic regression are illustrated at Table 4.
Finally, an additional analysis (Fig. 3) discovered the occurrence of satisfactory positive linear corellation between reporting quality and IF
[Pearson’s correlation (r = 0.740, p = 0.004)].
Discussion
CONSORT adherence
The present study evaluated the reporting quality of RCTsthat examined the effect of vitamin D supplement in thyroid autoimmunity according to 2010 CONSORT statement. The conclusion is that the overall CONSORT adherence is far from optimal, with the mean compliance equal to 61.15%. The number and sample size of RCTs based on our subjectis smaller than that of other endocrinological diseases probably due to rising interest of researchers in the last decade [35,36,37]. We collected and analyzed 13 articles referring to 1174 randomized participants. Only three of them showed compliance above 75%.
Furthermore, 16 of 37 checklist items (43.2%) were addressed by 75% or more. The report of crucial methodological characteristics like randomization (item 9: allocation concealment method—38%; item 10: implementation—7.7%) and blinding (item 11a: who was blinded—38%) was found to be suboptimal. Unclear or absent description of randomization and blinding degrades RCTs due to complicated risk of bias [38]. Also, inadequate explanation of adverse effects in their articles (item 19: harms or unintended effects—23%) will probably misguide the medical approach of the physicians and may even give wrong advice to their patients. Item 14b (Why the trial ended or was stopped—0%) was the least reported item.On the contrary, it is hopeful that significant items such as trial design (item 3a – 92%) and report of the interventions for each group (item 5–85%) achieved a strong representation.
Determinants of reporting quality
Univariate analysis suggested thatlarger sample size,higher number of authors,the presence of funding wereall associated with abetter reporting quality but not statistically significant. Only RCTs of high-ranked medical journals showed superior adherence to the CONSORT statement giving statistically significant results (p < 0.05) and additionally a strong linear correlation (r = 0.740). IF was previously studied and a number of studies demonstrated an important association between IF and reporting quality [28, 29, 32, 34]. This is because journals with a higher IF have more strictrules for the publication of studies.
Despite the indications of univariate analysis, logistic regressionof possible determining factors canceled the previous effectof impact factor in the reporting quality of RCTs.In any case, we have to make referenceto commercial funding. It is crucial that our study comes in harmony with previous showing non-significant impact in scientific information [28, 38,39,40].
In one hand, the reporting quality of RCTs for Vitamin D supplementation in autoimmune thyroid disorders appeared not to be affected by Covid-19 pandemic. On the other hand, several fields of research are being lured away from their main area of interest to the pandemic, including the possibility that other health topics are ignored or not done properly [41]. It is important to highlight that literature search involved three databases: PubMed/MEDLINE, Cochrane Library and Google scholar creating a source of 8196 studies and increasing the overall efficacy of search strategy. As is well known, CONSORT statement is free and the methodology of current study is easily accessible.
However, our results must be interpreted with skepticism and some points need to be addressed. Vitamin Dsupplementation in autoimmune thyroid disorders is not a field well studied by the research community. As a result, the number of RCTs we analyzed, is quiet low. Moreover, articles not published in English orreleased beyond the time limit were excluded. The researcher was not blinded to journal and all items were rated as equal. So, the methodological analysis becomes more susceptible to subjectivity as certain items like flow diagram, randomization and blinding are more important than others.
Considering the increasing number of publications, investigators are recommended to report their RCTs according to the CONSORT statement and the CONSORT statement should be implemented in the editorial process. The improvement of the quality of RCTs could assist to reach more conclusive results, to minimize biased conclusions, to elucidate better the clinical significance of RCTs, and to direct more specifically future medical research.
Conclusion
To the best of our knowledge, the present study is the first to evaluate the reporting quality of RCTs for Vitamin D supplementation in autoimmune thyroid disorders according to 2010 CONSORT statement. The results we obtained were discouraging. It is our feeling that our subject is generally badly reported. Taking into account the controversial role of VitD supplementation on the prevention and/or treatment of AITD and the increasing number of publications, we concluded that the compliance with CONSORT guidelines becomes essential in order to provide more reliable and consistent answers to scientific question.
Change history
20 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12020-023-03345-3
References
D.S.A. McLeod, D.S. Cooper, The incidence and prevalence of thyroid autoimmunity. Endocrine 42(2), 252–265 (2012)
R. Zhao, W. Zhang, C. Ma, Y. Zhao, R. Xiong, H. Wang et al. Immunomodulatory function of vitamin D and its role in autoimmune thyroid disease. Front. Immunol. 12, 574967 (2021)
C. Mele, M. Caputo, A. Bisceglia, M.T. Samà, M. Zavattaro, G. Aimaretti et al. Immunomodulatory effects of vitamin D in thyroid diseases. Nutrients 12(5), 1444 (2020)
M.T. Cantorna, Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc. Soc. Exp. Biol. Med. 223(3), 230–233 (2000)
Á. Gil, J. Plaza-Diaz, M.D. Mesa, Vitamin D: classic and novel actions. Ann. Nutr. Metab. 72(2), 87–95 (2018)
M. Bellan, G. Guzzaloni, M. Rinaldi, E. Merlotti, C. Ferrari, A. Tagliaferri et al. Altered glucose metabolism rather than naive type 2 diabetes mellitus (T2DM) is related to vitamin D status in severe obesity. Cardiovascular Diabetol. 13(1), 57 (2014)
C. di Somma, E. Scarano, L. Barrea, V. Zhukouskaya, S. Savastano, C. Mele et al. Vitamin D and neurological diseases: an endocrine view. Int. J. Mol. Sci. 18(11), 2482 (2017)
D. Gallo, L. Mortara, M.B. Gariboldi, S.A.M. Cattaneo, S. Rosetti, L. Gentile et al. Immunomodulatory effect of vitamin D and its potential role in the prevention and treatment of thyroid autoimmunity: a narrative review. J. Endocrinological Investig. 43(4), 413–429 (2020)
D. Kim, The role of vitamin D in Thyroid diseases. Int. J. Mol. Sci. 18(9), 1949 (2017)
S. Kivity, N. Agmon-Levin, M. Zisappl, Y. Shapira, E.V. Nagy, K. Dankó et al. Vitamin D and autoimmune thyroid diseases. Cell. Mol. Immunol. 8(3), 243–247 (2011)
G. Muscogiuri, D. Mari, S. Prolo, L. Fatti, M. Cantone, P. Garagnani et al. 25 hydroxyvitamin D deficiency and its relationship to autoimmune thyroid disease in the elderly. Int. J. Environ. Res. Public Health 13(9), 850 (2016)
D.Y. Shin, K.J. Kim, D. Kim, S. Hwang, E.J. Lee, Low serum vitamin D is associated with anti-thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med. J. 55(2), 476 (2014)
R. Goswami, R.K. Marwaha, N. Gupta, N. Tandon, V. Sreenivas, N. Tomar et al. Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: a community-based survey. Br. J. Nutr. 102(3), 382–386 (2009)
G. Muscogiuri, G. Tirabassi, G. Bizzaro, F. Orio, S.A. Paschou, A. Vryonidou et al. Vitamin D and thyroid disease: to D or not to D? Eur. J. Clin. Nutr. 69(3), 291–296 (2015)
G. Bizzaro, Y. Shoenfeld, Vitamin D and thyroid autoimmune diseases: the known and the obscure. Immunologic Res. 61(1–2), 107–109 (2015)
J. Yasmeh, F. Farpour, V. Rizzo, S. Kheradnam, I. Sachmechi, Hashimoto thyroiditis not associated with vitamin D deficiency. Endocr. Pract. 22(7), 809–813 (2016)
G. Effraimidis, K. Badenhoop, J.G.P. Tijssen, W.M. Wiersinga, Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur. J. Endocrinol. 167(1), 43–48 (2012)
S. Wang, Y. Wu, Z. Zuo, Y. Zhao, K. Wang, The effect of vitamin D supplementation on thyroid autoantibody levels in the treatment of autoimmune thyroiditis: a systematic review and a meta-analysis. Endocrine 59(3), 499–505 (2018)
J. Zhang, Y. Chen, H. Li, H. Li, Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: systematic review and meta-analysis. J. Int. Med. Res. 49(12), 030006052110606 (2021)
G. Bonadonna, P. Valagussa, Influence of clinical trials on current treatment strategy for Hodgkin’s disease. Int. J. Radiat. Oncol.*Biol.Phys. 19(1), 209–218 (1990)
H.O. Stolberg, G. Norman, I. Trop, Randomized controlled trials. Am. J. Roentgenol. 183(6), 1539–1544 (2004)
K.F. Schulz, Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA: J. Am. Med. Assoc. 273(5), 408–412 (1995)
C. Begg, Improving the quality of reporting of randomized controlled trials. JAMA 276(8), 637 (1996)
D. Moher, The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 285(15), 1987 (2001) Apr 18
D. Moher, S. Hopewell, K.F. Schulz, V. Montori, P.C. Gøtzsche, P.J. Devereaux et al. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 10(1), 28–55 (2012)
A. Stevens, L. Shamseer, E. Weinstein, F. Yazdi, L. Turner, J. Thielman et al. Relation of completeness of reporting of health research to journals’ endorsement of reporting guidelines: systematic review. BMJ (Clin. Res. ed.) 348, g3804 (2014). Jun 25
D. Rikos, E. Dardiotis, A-M Aloizou, V. Siokas, E. Zintzaras, G.M. Hadjigeorgiou. Reporting Quality of Randomized Controlled Trials in Restless Legs Syndrome Based on the CONSORT Statement. Tremor and other hyperkinetic movements (New York, NY). 2019;9
M. Kodounis, I.N. Liampas, T.S. Constantinidis, V. Siokas, A.-F.A. Mentis, A.-M. Aloizou et al. Assessment of the reporting quality of double-blind RCTs for ischemic stroke based on the CONSORT statement. J. Neurological Sci. 415, 116938 (2020). Aug
I. Liampas, A. Chlinos, V. Siokas, A. Brotis, E. Dardiotis, Assessment of the reporting quality of RCTs for novel oral anticoagulants in venous thromboembolic disease based on the CONSORT statement. J. Thrombosis Thrombolysis 48(4), 542–553 (2019)
M. Elcivan, A. Kowark, M. Coburn, H. A. Hamou, B. Kremer, H. Clusmann, et al. A retrospective analysis of randomized controlled trials on traumatic brain injury: evaluation of CONSORT Item Adherence. Brain Sci. 11(11), 1504 (2021)
D. Rikos, E. Dardiotis, A.-M. Aloizou, V. Siokas, E. Zintzaras, G.M Hadjigeorgiou. Reporting Quality of Randomized Controlled Trials in Restless Legs Syndrome Based on the CONSORT Statement. Tremor and other hyperkinetic movements (New York, NY). 2019;9
E. Beneki, C. Vrysis, E. Zintzaras, C. Doxani, Analysis of the quality of reporting of randomized controlled trials in anticoagulant versus antiplatelet medication for venous thromboembolism prophylaxis as governed by the CONSORT statement. J. Thrombosis Thrombolysis 52(1), 138–147 (2021)
S. Hopewell, M. Clarke, D. Moher, E. Wager, P. Middleton, D.G. Altman et al. CONSORT for Reporting Randomized Controlled Trials in Journal and Conference Abstracts: Explanation and Elaboration. PLoS Med. 5(1), e20 (2008)
D.C. Ziogas, E. Zintzaras, Analysis of the quality of reporting of randomized controlled trials in acute and chronic myeloid leukemia, and myelodysplastic syndromes as governed by the CONSORT statement. Ann. Epidemiol. 19(7), 494–500 (2009)
L. García-Molina, A.M. Lewis-Mikhael, B. Riquelme-Gallego, N. Cano-Ibáñez, M.J. Oliveras-López, A. Bueno-Cavanillas, Improving type 2 diabetes mellitus glycaemic control through lifestyle modification implementing diet intervention: a systematic review and meta-analysis. Eur. J. Nutr. 59(4), 1313–28. (2020)
E. Sainsbury, N.V. Kizirian, S.R. Partridge, T. Gill, S. Colagiuri, A.A. Gibson, Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res Clin. Pr. 139, 239–252 (2018)
G. Formoso, E. Perrone, S. Maltoni, S. Balduzzi, J. Wilkinson, V. Basevi, et al. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database Syst. Rev. 10(10), CD008536 (2016)
H. Balshem, M. Helfand, H.J. Schünemann, A.D. Oxman, R. Kunz, J. Brozek et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64(4), 401–406 (2011)
L. Saric, K. Vucic, K. Dragicevic, M. Vrdoljak, D. Jakus, I. Vuka et al. Comparison of conference abstracts and full-text publications of randomized controlled trials presented at four consecutive World Congresses of Pain: Reporting quality and agreement of results. Eur. J. Pain. (Lond., Engl.) 23(1), 107–116 (2019)
F. Song, S. Parekh, L. Hooper, Y.K. Loke, J. Ryder, A.J. Sutton et al. Dissemination and publication of research findings: an updated review of related biases. Health Technol. Assess. (Winch., Engl.) 14(8), 1–193 (2010)
M. Pai, Covidization of research: what are the risks? Nat. Med 26(8), 1159–1159 (2020)
R. Chahardoli, A.-A. Saboor-Yaraghi, A. Amouzegar, D. Khalili, A.Z. Vakili, F. Azizi, Can supplementation with vitamin D modify thyroid autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and thyroid profile (T3, T4, TSH) in Hashimoto’s thyroiditis? a double blind, randomized clinical trial. Horm. Metab. Res. = Horm.- und Stoffwechselforschung = Hormones et. Metab. 51(5), 296–301 (2019)
M. Nodehi, A. Ajami, M. Izad, H. AsgarianOmran, R. Chahardoli, A. Amouzegar et al. Effects of vitamin D supplements on frequency of CD4+ T-cell subsets in women with Hashimoto’s thyroiditis: a double-blind placebo-controlled study. Eur. J. Clin. Nutr. 73(9), 1236–1243 (2019)
P. VahabiAnaraki, A. Aminorroaya, M. Amini, F. Momeni, A. Feizi, B. Iraj et al. Effect of Vitamin D deficiency treatment on thyroid function and autoimmunity markers in Hashimoto’s thyroiditis: A double-blind randomized placebo-controlled clinical trial. J. Res. Med. Sci.: Off. J. Isfahan Univ. Med. Sci. 22, 103 (2017)
P.V. Anaraki, A. Aminorroaya, M. Amini, A. Feizi, B. Iraj, A. Tabatabaei, Effects of Vitamin D deficiency treatment on metabolic markers in Hashimoto thyroiditis patients. J. Res. Med. Sci.: Off. J. Isfahan Univ. Med. Sci. 22, 5 (2017)
Y. Simsek, I. Cakır, M. Yetmis, O.S. Dizdar, O. Baspinar, F. Gokay, Effects of Vitamin D treatment on thyroid autoimmunity. J. Res. Med. Sci.: Off. J. Isfahan Univ. Med. Sci. 21, 85 (2016)
S. Chaudhary, D. Dutta, M. Kumar, S. Saha, S.A. Mondal, A. Kumar, et al. Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Ind. J. Endocrinol. Metab. 20(3), 391–398 (2016)
K. K. Behera, G. K. Saharia, D. Hota, D. P. Sahoo, M. Sethy, A. Srinivasan, Effect of vitamin D supplementation on thyroid autoimmunity among subjects of autoimmune thyroid disease in a coastal province of india: a randomized open-label trial. Niger Med. J. 61(5), 237–240 (2020)
D. Grove-Laugesen, S. Malmstroem, E. Ebbehoj, A.L. Riis, T. Watt, K.W. Hansen et al. Effect of 9 months of vitamin D supplementation on arterial stiffness and blood pressure in Graves’ disease: a randomized clinical trial. Endocrine 66(2), 386–397 (2019)
D. Grove-Laugesen, P.K. Cramon, S. Malmstroem, E. Ebbehoj, T. Watt, K.W. Hansen et al. Effects of supplemental vitamin D on muscle performance and quality of life in graves’ disease: a randomized clinical trial. Thyroid.: Off. J. Am. Thyroid. Assoc. 30(5), 661–671 (2020)
X. Mei, J. Zeng, W.-X. Dai, H.-L. Yang, Y. Li, M.-W. Tang et al. Prevalence of hyperthyroidism with hypercalcemia in Xindu district and the efficacy of vitamin D3 treatment in these patients: a randomized trial. Ann. Palliat. Med. 10(9), 9640–9649 (2021)
K.V. Knutsen, A.A. Madar, M. Brekke, H.E. Meyer, Å.R. Eggemoen, I. Mdala et al. Effect of vitamin D on thyroid autoimmunity: a randomized, double-blind, controlled trial among ethnic minorities. J. Endocr. Soc. 1(5), 470–479 (2017)
D. Purnamasari, S. Djauzi, S. Setiati, A. Harahap, T. GdePemayun, J. Prihartono et al. Effects of oral alfacalcidol on maturation of dendritic cells in graves’ disease patients: a double-blinded randomized clinical trial. Asian J. Pharm. Clin. Res. 10(6), 100 (2017)
B. Ucan, M. Sahin, M. Sayki Arslan, N. Colak Bozkurt, M. Kizilgul, A. Güngünes et al. Vitamin D treatment in patients with hashimoto’s thyroiditis may decrease the development of hypothyroidism. Int. J. Vitam. Nutr. Res. InternationaleZeitschrift fur Vitam.- und Ernahrungsforschung J. Int. de. vitaminologie et. de. Nutr. 86(1–2), 9–17 (2016)
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by C.V., E.B., E.Z. and C.D. The first draft of the manuscript was written by Vrysis Christos and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vrysis, C., Beneki, E., Zintzaras, E. et al. Assessment of the reporting quality of randomised controlled trials for vitamin D supplementation in autoimmune thyroid disorders based on the CONSORT statement. Endocrine 80, 346–354 (2023). https://doi.org/10.1007/s12020-022-03270-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03270-x