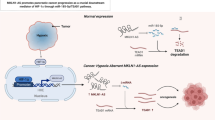
Abstract
Purpose
In this study, we evaluated the biological role of miRNA-31-5p in papillary thyroid cancer (PTC).
Methods
By using the real-time PCR, we measured miRNA-31-5p expression levels in 25 PTC tissues and in two human PTC cell lines (K1 and TPC-1). Then, K1 cells were transiently transfected with mirVana inhibitor or mirVana mimic to miRNA-31-5-p. Cell proliferation was determined by MTT and colony formation assays. The in vitro metastatic ability of thyroid cancer cells was evaluated by adhesion, migration and invasion assays. Epithelial mesenchymal transition (EMT) and Hippo pathway related gene and protein levels were evaluated by using the TaqMan™ Gene Expression Assays and western blot analysis, respectively.
Results
We found a significant increase of miR-31-5-p expression in tumor tissue and in K1 cells harboring the BRAF p.V600E mutation. Knockdown of miR-31-5p determined a reduction of cell proliferation, associated with a significant decrease in cell adhesion, migration and invasion properties. A downregulation of EMT markers and YAP/β-catenin axis was also observed.
Conclusions
Our findings suggest that miRNA-31-5p acts as oncogenic miRNA in human thyrocytes and its overexpression may be involved in the BRAF-related tumorigenesis in PTCs, providing new understanding into its pathological role in PTC progression and invasiveness.






Similar content being viewed by others
References
S. Bulotta, M. Celano, G. Costante, D. Russo, Novel therapeutic options for radioiodine-refractory thyroid cancer: redifferentiation and beyond. Curr. Opin. Oncol. 32(1), 13–19 (2020). https://doi.org/10.1097/CCO.0000000000000593
R. Niciporuka, J. Nazarovs, A. Ozolins, Z. Narbuts, E. Miklasevics, J. Gardovskis. Can we predict differentiated thyroid cancer behavior? role of genetic and molecular markers. Medicina (Kaunas, Lithuania) 57(10), 1131 (2021). https://doi.org/10.3390/medicina57101131.
M. Rogucki, A. Buczynska, A.J. Kretowski, A. Poplawska-Kita, The Importance of miRNA in the Diagnosis and Prognosis of Papillary Thyroid Cancer. J. Clin. Med. 10(20), 4738 (2021). https://doi.org/10.3390/jcm10204738
M. Hussain, Micro-RNAs (miRNAs): Genomic Organisation, Biogenesis and Mode of Action. Cell. Tissue Res. 349, 405–413 (2012). https://doi.org/10.1007/s00441-012-1438-0
C.R. Lima, C.C. Gomes, M.F. Santos, Role of microRNAs in endocrine cancer metastasis. Mol. Cell. Endocrinol. 456, 62–75 (2017). https://doi.org/10.1016/j.mce.2017.03.015
H. Shakib, S. Rajabi, M.H. Dehghan, F.J. Mashayekhi, N. Safari-Alighiarloo, M. Hedayati, Epithelial-to-mesenchymal transition in thyroid cancer: a comprehensive review. Endocrine 66, 435–455 (2019). https://doi.org/10.1007/s12020-019-02030-8
Y. Sun, S. Yu, Y. Liu, F. Wang, Y. Liu, H. Xiao, Expression of miRNAs in Papillary Thyroid Carcinomas Is Associated with BRAF Mutation and Clinicopathological Features in Chinese Patients. Int. J. Endocrinol. 2013, 128735 (2013). https://doi.org/10.1155/2013/128735
M. Papaioannou, A.G. Chorti, A. Chatzikyriakidou, K. Giannoulis, S. Bakkar, T.S. Papavramidis, MicroRNAs in Papillary Thyroid Cancer: What Is New in Diagnosis and Treatment. Front. Oncol. 11, 755097 (2022). https://doi.org/10.3389/fonc.2021.755097
M. Celano, F. Rosignolo, V. Maggisano, V. Pecce, M. Iannone, D. Russo, S. Bulotta, MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genomics. 2017, 6496570 (2017). https://doi.org/10.1155/2017/6496570
V. Maggisano, F. Capriglione, A. Verrienti, M. Celano, A. Gagliardi, S. Bulotta, M. Sponziello, C. Mio, V. Pecce, C. Durante, G. Damante, D. Russo, Identification of Exosomal microRNAs and Their Targets in Papillary Thyroid Cancer Cells. Biomedicines 10(5), 961 (2022). https://doi.org/10.3390/biomedicines10050961
Y. Wang, B.G. Liu, C.X. Zhou, MicroRNA-31 inhibits papillary thyroid carcinoma cell biological progression by directly targeting SOX11 and regulating epithelial-to-mesenchymal transition, ERK and Akt signaling pathways. Eur. Rev. Med. Pharmacol. Sci. 23, 5863–5873 (2019). https://doi.org/10.26355/eurrev_201907_18329
D. Yi, D. Zhang, J. He, Long non-coding RNA LIFR-AS1 suppressed the proliferation, angiogenesis, migration and invasion of papillary thyroid cancer cells via the miR-31-5p/SIDT2 axis. Cell. Cycle 20, 2619–2637 (2021). https://doi.org/10.1080/15384101.2021.1995129
R.M. Tuttle, B. Haugen, N.D. Perrier, Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 27(6), 751–756 (2017). https://doi.org/10.1089/thy.2017.0102
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G.W. Randolph, A.M. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D.L. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1–133 (2016). https://doi.org/10.1089/thy.2015.0020
N. Passon, E. Bregant, M. Sponziello, M. Dima, F. Rosignolo, C. Durante, M. Celano, D. Russo, S. Filetti, G. Damante, Somatic amplifications and deletions in genome of papillary thyroid carcinomas. Endocrine 50, 453–464 (2015). https://doi.org/10.1007/s12020-015-0592-z
R.E. Schweppe, J.P. Klopper, C. Korch, U. Pugazhenthi, M. Benezra, J.A. Knauf, J.A. Fagin, L.A. Marlow, J.A. Copland, R.C. Smallridge, B.R. Haugen, Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J. Clin. Endocrinol. Metab. 93, 4331–4341 (2008). https://doi.org/10.1210/jc.2008-1102
A. Bairoch, The Cellosaurus, a Cell-Line Knowledge Resource. J. Biomol. Tech. 29(2), 25–38 (2018). https://doi.org/10.7171/jbt.18-2902-002
V. Maggisano, M. Celano, S.M. Lepore, M. Sponziello, F. Rosignolo, V. Pecce, A. Verrienti, F. Baldan, C. Mio, L. Allegri, M. Maranghi, R. Falcone, G. Damante, D. Russo, S. Bulotta, Human telomerase reverse transcriptase in papillary thyroid cancer: gene expression, effects of silencing and regulation by BET inhibitors in thyroid cancer cells. Endocrine 63, 545–553 (2019). https://doi.org/10.1007/s12020-018-01836-2
K.J. Livak, S.J. Flood, J. Marmaro, W. Giusti, K. Deetz, Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 4, 357–362 (1995). https://doi.org/10.1101/gr.4.6.357
M. Celano, V. Maggisano, S. Bulotta, L. Allegri, V. Pecce, L. Abballe, G. Damante, D. Russo, Quercetin improves the effects of sorafenib on growth and migration of thyroid cancer cells. Endocrine 67, 496–498 (2020). https://doi.org/10.1007/s12020-019-02140-3
S. Borowicz, M. Van Scoyk, S. Avasarala, M.K.K. Rathinam, J. Tauler, R.K. Bikkavilli, R.A. Winn, The soft agar colony formation assay. J. Vis. Exp. 92, e51998 (2014). https://doi.org/10.3791/51998
S. Bulotta, M.V. Ierardi, J. Maiuolo, M.G. Cattaneo, A. Cerullo, L.M. Vicentini, N. Borgese, Basal nitric oxide release attenuates cell migration of HeLa and endothelial cells. Biochem. Biophys. Res. Commun. 386, 744e749 (2009). https://doi.org/10.1016/j.bbrc.2009.06.118
M. D’Agostino, P. Voce, M. Celano, M. Sponziello, S. Moretti, V. Maggisano, A. Verrienti, C. Durante, S. Filetti, E. Puxeddu, D. Russo, Sunitinib exerts only limited effects on the proliferation and differentiation of anaplastic thyroid cancer cells. Thyroid 22, 138–144 (2012). https://doi.org/10.1089/thy.2011.0060
S. Bulotta, A. Cerullo, R. Barsacchi, C. De Palma, D. Rotiroti, E. Clementi, N. Borgese, Endothelial nitric oxide synthase is segregated from caveolin-1 and localizes to the leading edge of migrating cells. Exp. Cell Res. 312(6), 877–889 (2006). https://doi.org/10.1016/j.yexcr.2005.12.014
N. Mastronikolis, E. Tsiambas, D. Roukas, P. Fotiades, A. Chrysovergis, V. Papanikolaou, E. Kyrodimos, S. Mastronikoli, A. Niotis, V. Ragos, Micro-RNAs signatures in papillary thyroid carcinoma. J. Buon. 25(5), 2144–2146 (2020)
X. Chen, L. Zhong, X. Li, W. Liu, Y. Zhao, J. Li, Down-regulation of microRNA-31-5p inhibits proliferation and invasion of osteosarcoma cells through Wnt/β-catenin signaling pathway by enhancing AXIN1. Exp. Mol. Pathol. 108, 32–41 (2019). https://doi.org/10.1016/j.yexmp.2019.03.001
Z. Lu, Q. He, J. Liang, W. Li, Q. Su, Z. Chen, Q. Wan, X. Zhou, L. Cao, J. Sun, Y. Wu, L. Liu, X. Wu, J. Hou, K. Lian, A. Wang, miR-31-5p Is a Potential Circulating Biomarker and Therapeutic Target for Oral Cancer. Mol. Ther. Nucleic Acids 16, 471–480 (2019). https://doi.org/10.1016/j.omtn.2019.03.012
Y.L. Du, Y. Liang, G.Q. Shi, Y. Cao, J. Qiu, L. Yuan, Z. Yong, L. Liu, J. Li, LINC00689 participates in proliferation, chemoresistance and metastasis via miR-31-5p/YAP/beta-catenin axis in colorectal cancer. Exp. Cell. Res. 395, 112176 (2020). https://doi.org/10.1016/j.yexcr.2020.112176
F. Song, Z. Xuan, X. Yang, X. Ye, Z. Pan, Q. Fang, Identification of key microRNAs and hub genes in non-small-cell lung cancer using integrative bioinformatics and functional analyses. J. Cell. Biochem. 121, 2690–2703 (2020). https://doi.org/10.1002/jcb.29489
H.G. Vuong, A.M.A. Altibi, U.N.P. Duong, L. Hassell, Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin. Endocrinol. (Oxf.). 87, 411–417 (2017). https://doi.org/10.1111/cen.13413
V. Vasko, A.V. Espinosa, W. Scouten, H. He, H. Auer, S. Liyanarachchi, A. Larin, V. Savchenko, G.L. Francis, A. de la Chapelle, M. Saji, M.D. Ringel, Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc. Natl Acad. Sci. USA. 104, 2803–2808 (2007). https://doi.org/10.1073/pnas.0610733104
P. Baquero, E. Jimenez-Mora, A. Santos, M. Lasa, A. Chiloeches, TGF beta induces epithelial-mesenchymal transition of thyroid cancer cells by both the BRAF/MEK/ERK and Src/FAK pathways. Mol. Carcinog. 55, 1639–1654 (2016). https://doi.org/10.1002/mc.22415
M. Sponziello, F. Rosignolo, M. Celano, V. Maggisano, V. Pecce, R.F. De Rose, G.E. Lombardo, C. Durante, S. Filetti, G. Damante, D. Russo, S. Bulotta, Fibronectin-1 expression is increased in aggressive thyroid cancer and favors the migration and invasion of cancer cells. Mol. Cell. Endocrinol. 431, 123–132 (2016). https://doi.org/10.1016/j.mce.2016.05.007
S. Xia, C. Wang, E.L. Postma, Y. Yang, X. Ni, W. Zhan, Fibronectin 1 promotes migration and invasion of papillary thyroid cancer and predicts papillary thyroid cancer lymph node metastasis. Onco Targets Ther. 10, 1743–1755 (2017). https://doi.org/10.2147/OTT.S122009
Y. Lei, L. Chen, G. Zhang, A. Shan, C. Ye, B. Liang, J. Sun, X. Liao, C. Zhu, Y. Chen, J. Wang, E. Zhang, L. Deng, MicroRNAs target the Wnt/betacatenin signaling pathway to regulate epithelial mesenchymal transition in cancer (Review). Oncol. Rep. 44, 1299–1313 (2020). https://doi.org/10.3892/or.2020.7703
B. Mi, Q. Li, T. Li, G. Liu, J. Sai, High miR-31-5p expression promotes colon adenocarcinoma progression by targeting TNS1. Aging (Albany NY) 12, 7480–7490 (2020). https://doi.org/10.18632/aging.103096
I. Akrida, V. Bravou, H. Papadaki, The deadly cross-talk between Hippo pathway and epithelial-mesenchymal transition (EMT) in cancer. Mol. Biol. Rep. 49(10), 10065–10076 (2022). https://doi.org/10.1007/s11033-022-07590-z
T. Pei, Y. Li, J. Wang, H. Wang, Y. Liang, H. Shi, B. Sun, D. Yin, J. Sun, R. Song, S. Pan, Y. Sun, H. Jiang, T. Zheng, L. Liu, YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget 6, 17206–17220 (2015). https://doi.org/10.18632/oncotarget.4043
S.E. Hiemer, L. Zhang, V.K. Kartha, T.S. Packer, M. Almershed, V. Noonan, M. Kukuruzinska, M.V. Bais, S. Monti, X. Varelas, A YAP/TAZ-Regulated Molecular Signature Is Associated with Oral Squamous Cell Carcinoma. Mol. Cancer Res. 13, 957–968 (2015). https://doi.org/10.1158/1541-7786.MCR-14-0580
W. Zhang, Y. Gao, F. Li, X. Tong, Y. Ren, X. Han, S. Yao, F. Long, Z. Yang, H. Fan, L. Zhang, H. Ji, YAP promotes malignant progression of Lkb1-deficient lung adenocarcinoma through downstream regulation of survivin. Cancer Res. 75, 4450–4457 (2015). https://doi.org/10.1158/0008-5472.CAN-14-3396
M. Celano, C. Mignogna, F. Rosignolo, M. Sponziello, M. Iannone, S.M. Lepore, G.E. Lombardo, V. Maggisano, A. Verrienti, S. Bulotta, C. Durante, C. Di Loreto, G. Damante, D. Russo, Expression of YAP1 in aggressive thyroid cancer. Endocrine 59(1), 209–212 (2018). https://doi.org/10.1007/s12020-017-1240-6
Z. Liu, W. Zeng, Y. Maimaiti, J. Ming, Y. Guo, Y. Liu, C. Liu, T. Huang, High Expression of Yes-activated Protein-1 in Papillary Thyroid Carcinoma Correlates With Poor Prognosis. Appl. Immunohistochem. Mol. Morphol. 27(1), 59–64 (2019). https://doi.org/10.1097/PAI.0000000000000544.
M. Wang, M. Dai, D. Wang, W. Xiong, Z. Zeng, C. Guo, The regulatory networks of the Hippo signaling pathway in cancer development. J. Cancer 12, 6216–6230 (2021). https://doi.org/10.7150/jca.62402
P. Ma, J. Han, Overexpression of miR-100-5p inhibits papillary thyroid cancer progression via targeting FZD8. Open Med. (Wars.) 17(1), 1172–1182 (2022). https://doi.org/10.1515/med-2022-0490
B. Basu, M.K. Ghosh, Ubiquitination and deubiquitination in the regulation of epithelial-mesenchymal transition in cancer: Shifting gears at the molecular level. Biochim. Biophys. Acta Mol. Cell. Res. 1869, 119261 (2022). https://doi.org/10.1016/j.bbamcr.2022.119261
Acknowledgements
This paper was financially supported by the Department of Health Sciences, University of Catanzaro “Magna Græcia”.
Funding
This study was funded by grants from the Italian Ministry of Universities and Research, PRIN2017EKMFTN_003.
Author information
Authors and Affiliations
Contributions
Conceptualization, V.M., F.C., and D.R.; Investigation, V.M., F.C., A.V., and M.C.; Data curation, A.V., M.S., V.P., M.C.; Writing-original draft preparation, V.M., S.B., and D.R.; Writing-review and editing, C.D., S.B., and D.R.; Supervision, C.D., D.R., and S.B. All authors have read and agreed to the published version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was conducted according to guidelines of the Declaration of Helsinki and approved by the ethics committee of Sapienza University of Rome (protocol code 1184/17 and date of approval 21/12/2017).
Consent for publication
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maggisano, V., Capriglione, F., Verrienti, A. et al. Expression of miR-31-5p affects growth, migration and invasiveness of papillary thyroid cancer cells. Endocrine 79, 517–526 (2023). https://doi.org/10.1007/s12020-022-03267-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03267-6