Abstract
Background
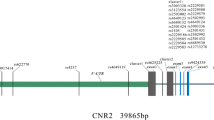
Genetic factors play a critical role in the pathogenesis of osteoporosis. The imbalance of WNT/β-catenin will cause the occurrence of osteoporosis. LRP5 and AXIN1 play an important role in the classical Wnt/β-catenin signaling pathway. Our study was aimed to determine the association between five candidate single nucleotide polymorphisms (SNPs) of LRP5 or AXIN1 and osteoporosis susceptibility in Chinese Han population.
Methods
A total of 599 osteoporosis patients and 599 healthy individuals were recruited for this case-control study. Agena MassARRAY was used to genotype SNPs. The association between SNPs and osteoporosis susceptibility in different genetic models was analyzed by PLINK software. We used false-positive report probability (FPRP) analysis to detect whether the positive results were just chance or noteworthy observations. Multifactor dimension reduction (MDR) was used to analyze the interaction of SNP–SNP in the osteoporosis risk. Finally, haplotype analysis was performed by plink1.07 and Haploview software.
Results
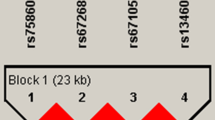
We found that LRP5 rs11228240, AXIN1 rs2301522, and rs9921222 were significantly associated with the osteoporosis susceptibility. The results of subgroup analysis showed that LRP5 rs11228240 (protective factor) and AXIN1 rs2301522 (risk factor) were associated with the susceptibility of osteoporosis among participants who were age >60 years, female or BMI ≤ 24; AXIN1 rs9921222 significantly increased the risk of osteoporosis among participants with BMI ≤ 24. The genotype Ars2301522Crs9921222 could increase the susceptibility of osteoporosis (p = 0.026). The rs11228219LPR5, rs11228240 LPR5, rs2301522AXIN1, and rs9921222AXIN1 four-site model was the best model for predicting the osteoporosis risk (test accuracy = 0.541; CVC = 10/10).
Conclusions
The LRP5-rs11228240, AXIN1-rs2301522, and AXIN1- rs9921222 were associated with osteoporosis susceptibility in Chinese Han population.



Similar content being viewed by others
References
B. Levi, M.T. Longaker, Concise review: adipose-derived stromal cells for skeletal regenerative medicine. Stem Cells 29(4), 576–582 (2011)
F. Henjes, L. Lourido, C. Ruiz-Romero, J. Fernández-Tajes, J.M. Schwenk, M. Gonzalez-Gonzalez et al. Analysis of autoantibody profiles in osteoarthritis using comprehensive protein array concepts. J. Proteom. Res. 13(11), 5218–5229 (2014)
M. Okubo, Y. Okada, [Destruction of the articular cartilage in osteoarthritis]. Clin. Calcium 23(12), 1705–1713 (2013)
S. Sampson, A. Botto-van Bemden, D. Aufiero, Autologous bone marrow concentrate: review and application of a novel intra-articular orthobiologic for cartilage disease. Phys. Sportsmed. 41(3), 7–18 (2013)
H.W. Deng, M.R. Stegman, K.M. Davies, T. Conway, R.R. Recker, Genetic determination of variation and covariation of peak bone mass at the hip and spine. J. Clin. Densitometr. 2(3), 251–263 (1999)
S. Otsuru, P.L. Gordon, K. Shimono, R. Jethva, R. Marino, C.L. Phillips et al. Transplanted bone marrow mononuclear cells and MSCs impart clinical benefit to children with osteogenesis imperfecta through different mechanisms. Blood. 120(9), 1933–1941 (2012)
M.H. Lim, W.K. Ong, S. Sugii, The current landscape of adipose-derived stem cells in clinical applications. Expert Rev. Mol. Med. 16, e8 (2014)
X. Zhu, W. Bai, H. Zheng, Twelve years of GWAS discoveries for osteoporosis and related traits: advances, challenges and applications. Bone Res. 9(1), 23 (2021).
D.P. Kiel, S. Demissie, J. Dupuis, K.L. Lunetta, J.M. Murabito, D. Karasik, Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med. Genet. 8(Suppl 1), S14 (2007)
H.F. Zheng, V. Forgetta, Y.H. Hsu, K. Estrada, A. Rosello-Diez, P.J. Leo et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 526(7571), 112–117 (2015)
J.B. Richards, F. Rivadeneira, M. Inouye, T.M. Pastinen, N. Soranzo, S.G. Wilson et al. Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371(9623), 1505–1512 (2008)
L. Tian, H. Xiao, M. Li, X. Wu, Y. Xie, J. Zhou et al. A novel Sprouty4-ERK1/2-Wnt/β-catenin regulatory loop in marrow stromal progenitor cells controls osteogenic and adipogenic differentiation. Metabolism 105, 154189 (2020)
Y. Wang, U. Singhal, Y. Qiao, T. Kasputis, J.S. Chung, H. Zhao et al. Wnt Signaling Drives Prostate Cancer Bone Metastatic Tropism and Invasion. Transl. Oncol. 13(4), 100747 (2020)
S. Yuan, F. Tao, X. Zhang, Y. Zhang, X. Sun, D. Wu, Role of Wnt/β-catenin signaling in the chemoresistance modulation of colorectal cancer. Biomed Res Int 2020, 9390878 (2020).
Y.J. Zhou, P. Wang, H.Y. Chen, C. Liu, Q.D. Ji, X.T. Yang et al. [Effect of Pulsed Electromagnetic Fields on Osteogenic Differentiation and Wnt/β-catenin Signaling Pathway in Rat Bone Marrow Mesenchymal Stem Cells]. Sichuan da xue xue bao Yi xue ban = Journal of Sichuan University Medical science edition 46(3), 347–353 (2015)
F. Long, Targeting intercellular signals for bone regeneration from bone marrow mesenchymal progenitors. Cell Cycle. 7(14), 2106–2111 (2008)
A. Kamiński, M. Karasiewicz, A. Bogacz, K. Dziekan, A. Seremak-Mrozikiewicz, B. Czerny, The importance of the Wnt/β-catenin pathway and LRP5 protein in bone metabolism of postmenopausal women. Adv. Clin. Exp. Med. 28(2), 179–184 (2019)
D. Zhang, Z. Ge, X. Ma, L. Zhi, Y. Zhang, X. Wu et al. Genetic association study identified a 20 kb regulatory element in WLS associated with osteoporosis and bone mineral density in Han Chinese. Scientific Rep. 7(1), 13668 (2017)
W.F. Li, S.X. Hou, B. Yu, D. Jin, C. Férec, J.M. Chen, Genetics of osteoporosis: perspectives for personalized medicine. Personalized Med. 7(6), 655–668 (2010)
H.F. Zheng, J.H. Tobias, E. Duncan, D.M. Evans, J. Eriksson, L. Paternoster et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 8(7), e1002745 (2012)
U. Styrkarsdottir, G. Thorleifsson, S.A. Gudjonsson, A. Sigurdsson, J.R. Center, S.H. Lee et al. Sequence variants in the PTCH1 gene associate with spine bone mineral density and osteoporotic fractures. Nat. Commun. 7, 10129 (2016)
M.P. Yavropoulou, P. Kollia, D. Chatzidimitriou, S. Samara, L. Skoura, J.G. Yovos, Severe osteoporosis with multiple spontaneous vertebral fractures in a young male carrying triple polymorphisms in the vitamin D receptor, collagen type 1, and low-density lipoprotein receptor-related peptide 5 genes. Hormones. 15(4), 551–556 (2016)
X.Y. Jiang, Y. Chen, L. Xu, X. Li, F.F. Cao, L. Li, et al. Association of LPR5 polymorphism with bone mass density and cholesterol level in population of Chinese Han. Exp. Clin. Endocrinol. Diabetes. 118(6), 388–391 (2010)
T. Mizuguchi, I. Furuta, Y. Watanabe, K. Tsukamoto, H. Tomita, M. Tsujihata et al. LRP5, low-density-lipoprotein-receptor-related protein 5, is a determinant for bone mineral density. J. Hum. Genet. 49(2), 80–86 (2004)
G.L. Lin, K.D. Hankenson, Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell Biochem. 112(12), 3491–3501 (2011)
Y.J. Liu, L. Zhang, C.J. Papasian, H.W. Deng, Genome-wide Association Studies for Osteoporosis: A 2013 Update. J. Bone Metab. 21(2), 99–116 (2014)
M.A. Rosales-Reynoso, A.M. Saucedo-Sariñana, K.B. Contreras-Díaz, R.M. Márquez-González, P. Barros-Núñez, T.D. Pineda-Razo et al. Genetic polymorphisms in APC, DVL2, and AXIN1 are associated with susceptibility, advanced TNM stage or tumor location in colorectal cancer. Tohoku J. Exp. Med. 249(3), 173–183 (2019)
L.A. Armas, R.R. Recker, Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol. Metab. Clin. N. Am. 41(3), 475–486 (2012)
C.J. Crandall, V.O. Yildiz, J. Wactawski-Wende, K.C. Johnson, Z. Chen, S.B. Going et al. Postmenopausal weight change and incidence of fracture: post hoc findings from Women’s Health Initiative Observational Study and Clinical Trials. BMJ. 350, h25 (2015)
O. de Matos, D.J. Lopes da Silva, J. Martinez de Oliveira, C. Castelo-Branco, Effect of specific exercise training on bone mineral density in women with postmenopausal osteopenia or osteoporosis. Gynecol. Endocrinol. 25(9), 616–620 (2009)
A.M. Parfitt, Z.H. Han, S. Palnitkar, D.S. Rao, M.S. Shih, D. Nelson, Effects of ethnicity and age or menopause on osteoblast function, bone mineralization, and osteoid accumulation in iliac bone. J. Bone Miner. Res. 12(11), 1864–1873 (1997)
V. Krishnan, H.U. Bryant, O.A. Macdougald, Regulation of bone mass by Wnt signaling. J. Clin. Investig. 116(5), 1202–1209 (2006)
J.J. Westendorf, R.A. Kahler, T.M. Schroeder, Wnt signaling in osteoblasts and bone diseases. Gene. 341, 19–39 (2004)
V.S. Li, S.S. Ng, P.J. Boersema, T.Y. Low, W.R. Karthaus, J.P. Gerlach et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 149(6), 1245–1256 (2012)
W. Huang, Q. Li, M. Amiry-Moghaddam, M. Hokama, S.H. Sardi, M. Nagao et al. Critical endothelial regulation by LRP5 during retinal vascular development. PloS One 11(3), e0152833 (2016)
C.C. Malbon, Wnt signalling: the case of the ‘missing’ G-protein. Biochem. J. 433(3), e3–e5 (2011)
G. Haugeberg, R.E. Ørstavik, T. Uhlig, J.A. Falch, J.I. Halse, T.K. Kvien, Comparison of ultrasound and X-ray absorptiometry bone measurements in a case control study of femaler heumatoid arthritis patients and randomly selected subjects in the population. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. Osteoporos Int. 14(4), 312–319 (2003)
L.Q. Bian, R.Z. Li, Z.Y. Zhang, Y.J. Jin, H.W. Kang, Z.Z. Fang et al. Effects of total bilirubin on the prevalence of osteoporosis in postmenopausal women without potential liver disease. J. Bone Miner. Metab. 31(6), 637–643 (2013)
S.M. Kweon, D.H. Sohn, J.H. Park, J.H. Koh, E.K. Park, H.N. Lee et al. Male patients with rheumatoid arthritis have an increased risk of osteoporosis: frequency and risk factors. Medicine. 97(24), e11122 (2018)
J. An, H. Yang, Q. Zhang, C. Liu, J. Zhao, L. Zhang et al. Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci.147, 46–58 (2016)
Acknowledgements
We thank all authors for their contributions and supports. We are also grateful to all participants for providing blood samples.
Author contributions
Conceptualization, K.W. and C.Z.; methodology, Y.C.; software, Y.C. and X.H.; data curation, Y.C. and X.H.; writing, review and editing, Y.C.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent to publication
All the authors agreed to publish the manuscript.
Ethics approval and consent to participate
This study was conducted under the standard approved by the the Second Affiliated Hospital of Xi’an Jiaotong University, and conformed to the ethical principles for medical research involving humans of the World Medical Association Declaration of Helsinki. All participants signed informed consent forms before participating in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Cui, Y., Hu, X., Zhang, C. et al. The genetic polymorphisms of key genes in WNT pathway (LRP5 and AXIN1) was associated with osteoporosis susceptibility in Chinese Han population. Endocrine 75, 560–574 (2022). https://doi.org/10.1007/s12020-021-02866-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02866-z