Abstract
Introduction
Vandetanib is indicated for adults with advanced medullary thyroid cancer (MTC).
Objectives
To describe the efficacy and toxicity profile of vandetanib treatment with a maximal follow-up of 11 years at Institut Gustave Roussy/France.
Methods
A review of the clinical files of the 76 MTC patients treated with vandetanib. Efficacy was estimated by markers and imaging.
Results
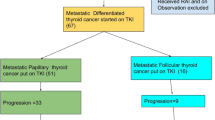
A total of 76 patients received vandetanib. Nine were excluded from efficacy analysis because lack of morphological data. The overall (N = 76) median treatment duration was 17.6 (range: 0.7–130.6) months and the median progression-free survival (PFS) was 22.7 (95% CI, 13.9–37.3) months. In total, 21/76 (27.6%) patients were classified as long-term users because have received vandetanib for more than 48 months, with a median treatment duration of 68.1 (range: 49.1–130.6) months. For long-term vandetanib users, the objective response rate was 85.7%, the median time to best response was 27.8 (11.6.1–110) months and the median duration of response was 70.4 (38.3–127.5) (95% CI 49.5–102.8) months with a median PFS of 73.2 (95% CI, 53.1–105.6) months. Duration of response had a significant negative correlation with patient age at diagnosis (p = 0.03) and was significantly higher in patients that did not have confirmed tumor progression before treatment onset (p = 0.007). After 48 months of vandetanib use, renal failure took place in two patients and heart failure, cholecystitis, acute pancreatitis, posterior encephalopathy, and skin cancer first occurred in one patient, each.
Conclusions
Our findings suggest that a substantial number of patients receiving first-/second-line vandetanib may sustain long clinical benefit and that a younger age at diagnosis and the absence of progression before treatment could be considered as predictors of durable response.




Similar content being viewed by others
References
L. Ceolin, M.A. Duval, S. da, A.F. Benini, C.V. Ferreira, A.L. Maia, Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr. Relat. Cancer 26, R499–518 (2019)
S.A. Wells et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J. Clin. Oncol. 30, 134–141 (2012)
J. Hadoux, M. Schlumberger, Chemotherapy and tyrosine-kinase inhibitors for medullary thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 31, 335–347 (2017)
C. Resteghini et al. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract. Res. Clin. Endocrinol. Metab. 31, 349–361 (2017)
C.N. Chougnet et al. Vandetanib for the treatment of advanced medullary thyroid cancer outside a clinical trial: results from a french cohort. Thyroid 25, 386–391 (2015)
D. Viola, R. Elisei, Management of medullary thyroid cancer. Endocrinol. Metab. Clin. N. Am. 48, 285–301 (2019)
M.E. Cabanillas, M. Ryder, C. Jimenez, Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr. Rev. 40, 1573–1604 (2019)
S.R. Priya, C.S. Dravid, R. Digumarti, M. Dandekar, Targeted therapy for medullary thyroid cancer: a review. Front. Oncol. 7, 238 (2017). https://doi.org/10.3389/fonc.2017.00238
G. Geller, J. Laskin, W.Y. Cheung, C. Ho, A retrospective review of the multidisciplinary management of medullary thyroid cancer: eligibility for systemic therapy. Thyroid Res. 10, 6 (2017)
C. Romei et al. New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations. J. Med. Genet. 53, 729–734 (2016)
C. Romei et al. Twenty years of lesson learning: how does the RET genetic screening test impact the clinical management of medullary thyroid cancer? Clin. Endocrinol. 82, 892–899 (2015)
L. Valerio et al. Medullary thyroid cancer treated with vandetanib: predictors of a longer and durable response. Endocr. Relat. Cancer 27, 97–110 (2020)
W.-X. Qi, A.-N. He, Z. Shen, Y. Yao, Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis. Br. J. Clin. Pharmacol. 76, 348–357 (2013)
R. Milling et al. Pazopanib, cabozantinib, and vandetanib in the treatment of progressive medullary thyroid cancer with a special focus on the adverse effects on hypertension. Int. J. Mol. Sci. 19, 3258 (2018)
M.S. Brose et al. Management of treatment-related toxicities in advanced medullary thyroid cancer. Cancer Treat. Rev. 66, 64–73 (2018)
Acknowledgements
Thiago Magalhães da Silva is professor at Department of biological sciences, Southwestern State University of Bahia, Jequié, Bahia, Brazil.
Funding
H.E.R. had his postdoctoral fellowship funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Federal University of Bahia (UFBA); this research did not receive any other specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.S. and S.L. have attended advisory boards for Astra Zeneca. M.S. received research funding from Astra Zeneca, Amgen, Exelixis, Bayer, and Eisai. The other authors have declared no conflicts of interest.
Informed consent
Informed consent has been obtained from all the patients for publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ramos, H.E., Hecht, F., Berdelou, A. et al. Long-term follow-up and safety of vandetanib for advanced medullary thyroid cancer. Endocrine 71, 434–442 (2021). https://doi.org/10.1007/s12020-020-02426-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02426-x