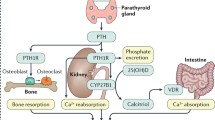
Abstract
The aim of this clinical narrative review is to summarize and critically appraise the literature on the differential diagnosis of hypocalcemia and to provide its correct management. Calcium is essential for muscle contraction and neurotransmitter release, but clinical manifestations of hypocalcaemia (serum calcium level <8 mg/dl; 2.12 mmol/L) may involve almost any organ and system and may range from asymptomatic to life-threating conditions. Disorders causing hypocalcemia can be divided into parathyroid hormone (PTH) and non-PTH mediated. The most frequent cause of hypocalcemia is postsurgical hypoparathyroidism, while a more comprehensive search for other causes is needed for appropriate treatment in the non PTH-mediated forms. Intravenous calcium infusion is essential to raise calcium levels and resolve or minimize symptoms in the setting of acute hypocalcemia. Oral calcium and/or vitamin D supplementation is the most frequently used as treatment of chronic hypocalcemia. In hypoparathyroidism, providing the missing hormone with the use of the recombinant human (rh) PTH(1–84) has been recently approved both by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). This new therapy has the advantage of being effective for correcting serum calcium levels and significantly reducing the daily requirements of calcium and active vitamin D supplements. However, due to the high cost, a strict selection of candidates to this therapy is necessary. More challenging is the long-term hypocalcemia treatment, due to its associated complications. The development of long-acting recombinant human PTH will probably modify the management of chronic hypoparathyroidism in the future.

Similar content being viewed by others
References
R.B. Payne, A.J. Little, R.B. Williams, J.R. Milner, Interpretation of serum calcium in patients with abnormal serum proteins. Br. Med. J. 4(5893), 643–646 (1973). https://doi.org/10.1136/bmj.4.5893.643
F.R. Bringhurst, D.M., Kronenberg, Bone and mineral metabolism in health and disease. In: Larry, J. (ed.) Harrison's principles of internal medicine, McGraw-Hill, New York, NY (Harrison's Principles of Internal Medicine, 20 Eds)
F.M. Hannan, R.V. Thakker, Investigating hypocalcaemia. Bmj 346, f2213 (2013). https://doi.org/10.1136/bmj.f2213
S. Minisola, J. Pepe, S. Piemonte, C. Cipriani, The diagnosis and management of hypercalcaemia. Bmj 350, h2723 (2015). https://doi.org/10.1136/bmj.h2723
F. Ferrone, J. Pepe, V.C. Danese, V. Fassino, V. Cecchetti, F. De Lucia, F. Biamonte, L. Colangelo, G. Ferrazza, E. Panzini, A. Scillitani, L. Nieddu, F. Blocki, S.D. Rao, S. Minisola, C. Cipriani, The relative influence of serum ionized calcium and 25-hydroxyvitamin D in regulating PTH secretion in healthy subjects. Bone 125, 200–206 (2019). https://doi.org/10.1016/j.bone.2019.05.029
B.L. Clarke, E.M. Brown, M.T. Collins, H. Juppner, P. Lakatos, M.A. Levine, M.M. Mannstadt, J.P. Bilezikian, A.F. Romanischen, R.V. Thakker, Epidemiology and diagnosis of hypoparathyroidism. J. Clin. Endocrinol. Metab. 101(6), 2284–2299 (2016). https://doi.org/10.1210/jc.2015-3908
C. Cipriani, J. Pepe, F. Biamonte, R. Manai, P. Biondi, L. Nieddu, L. Cianferotti, M.L. Brandi, S. Minisola, The epidemiology of hypoparathyroidism in Italy: an 8-year register-based study. Calcif. tissue Int. 100(3), 278–285 (2017). https://doi.org/10.1007/s00223-016-0222-7
E. Bove-Fenderson, M. Mannstadt, Hypocalcemic disorders. Best practice & research. Clin. Endocrinol. Metab. 32(5), 639–656 (2018). https://doi.org/10.1016/j.beem.2018.05.006
J.J. Diez, E. Anda, J. Sastre, B. Perez Corral, C. Alvarez-Escola, L. Manjon, M. Paja, M. Sambo, P. Santiago Fernandez, C. Blanco Carrera, J.C. Galofre, E. Navarro, C. Zafon, E. Sanz, A. Oleaga, O. Bandres, S. Donnay, A. Megia, M. Picallo, C. Sanchez Ragnarsson, G. Baena-Nieto, J.C.F. Garcia, B. Lecumberri, M.S. de la Vega, A.R. Romero-Lluch, P. Iglesias, Prevalence and risk factors for hypoparathyroidism following total thyroidectomy in Spain: a multicentric and nation-wide retrospective analysis. Endocrine 66(2), 405–415 (2019). https://doi.org/10.1007/s12020-019-02014-8
G.L. Canu, F. Medas, A. Longheu, F. Boi, G. Docimo, E. Erdas, P.G. Calo, Correlation between iPTH Levels on the First postoperative day after total thyroidectomy and permanent hypoparathyroidism: our experience. Open Med. 14, 437–442 (2019). https://doi.org/10.1515/med-2019-0047
L. Underbjerg, T. Sikjaer, L. Mosekilde, L. Rejnmark, Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J. Bone Miner. Res. 28(11), 2277–2285 (2013). https://doi.org/10.1002/jbmr.1979
A. Kelly, M.A. Levine, Hypocalcemia in the critically ill patient. J. Inten. Care Med. 28(3), 166–177 (2013). https://doi.org/10.1177/0885066611411543
M.N. Rao, D.M. Shoback, Hypocalcemia. In: K.R. Feingold, B. Anawalt, A. Boyce, G. Chrousos, K. Dungan, A. Grossman, J.M. Hershman, G. Kaltsas, C. Koch, P. Kopp, M. Korbonits, R. McLachlan, J.E. Morley, M. New, L. Perreault, J. Purnell, R. Rebar, F. Singer, D.L. Trence, A. Vinik, D.P. Wilson (eds.) Endotext, South Dartmouth (MA) (2000)
D.M. Shoback, J.P. Bilezikian, A.G. Costa, D. Dempster, H. Dralle, A.A. Khan, M. Peacock, M. Raffaelli, B.C. Silva, R.V. Thakker, T. Vokes, R. Bouillon, Presentation of hypoparathyroidism: etiologies and clinical features. J. Clin. Endocrinol. Metab. 101(6), 2300–2312 (2016). https://doi.org/10.1210/jc.2015-3909
G. Marcucci, L. Cianferotti, M.L. Brandi, Clinical presentation and management of hypoparathyroidism. Best. Pract. Res. Clin. Endocrinol. Metab. 32(6), 927–939 (2018). https://doi.org/10.1016/j.beem.2018.09.007
I.A. Hujoel, The association between serum calcium levels and Chvostek sign: a population-based study. Neurol. Clin. Pract. 6(4), 321–328 (2016). https://doi.org/10.1212/CPJ.0000000000000270
C.P.M. Heemskerk, M. Pereboom, K. van Stralen, F.A. Berger, P. van den Bemt, A.F.M. Kuijper, R.T.M. van der Hoeven, A.K. Mantel-Teeuwisse, M.L. Becker, Risk factors for QTc interval prolongation. Eur. J. Clin. Pharm. 74(2), 183–191 (2018). https://doi.org/10.1007/s00228-017-2381-5
M. Duval, K. Bach, D. Masson, C. Guimard, P. Le Conte, D. Trewick, Is severe hypocalcemia immediately life-threatening? Endocr. Connect (2018). https://doi.org/10.1530/EC-18-0267
J.C. Jentzer, S. Vallabhajosyula, A.K. Khanna, L.S. Chawla, L.W. Busse, K.B. Kashani, Management of refractory vasodilatory shock. Chest 154(2), 416–426 (2018). https://doi.org/10.1016/j.chest.2017.12.021
S. Minisola, C. Cipriani, L. Colangelo, F. Biamonte, J. Pepe, Serum calcium values and refractory vasodilatory shock. Chest 155(1), 242 (2019). https://doi.org/10.1016/j.chest.2018.08.1066
P.V. Jariwala, B. Sudarshan, M.S. Aditya, L. Praveer, K.S. Chandra, Hypoparathyroidism–a cause of reversible dilated cardiomyopathy. J. Assoc. Physicians India 58, 500–502 (2010)
D.M. Mitchell, S. Regan, M.R. Cooley, K.B. Lauter, M.C. Vrla, C.B. Becker, S.A. Burnett-Bowie, M. Mannstadt, Long-term follow-up of patients with hypoparathyroidism. J. Clin. Endocrinol. Metab. 97(12), 4507–4514 (2012). https://doi.org/10.1210/jc.2012-1808
R. Nardone, F. Brigo, E. Trinka, Acute symptomatic seizures caused by electrolyte disturbances. J. Clin. Neurol. 12(1), 21–33 (2016). https://doi.org/10.3988/jcn.2016.12.1.21
P. Han, B.J. Trinidad, J. Shi, Hypocalcemia-induced seizure: demystifying the calcium paradox. ASN Neuro. 7(2) (2015). https://doi.org/10.1177/1759091415578050
E. Iacovelli, F. Gilio, M.L. Mascia, A. Scillitani, E. Romagnoli, F. Pichiorri, S. Fucile, S. Minisola, M. Inghilleri, Acute and chronic effects of hypercalcaemia on cortical excitability as studied by 5 Hz repetitive transcranial magnetic stimulation. J. Physiol. 589, 1619–1626 (2011). https://doi.org/10.1113/jphysiol.2010.201111
C.G. Phillips, M.T. Harnett, W. Chen, S.M. Smith, Calcium-sensing receptor activation depresses synaptic transmission. J. Neurosci. 28(46), 12062–12070 (2008). https://doi.org/10.1523/JNEUROSCI.4134-08.2008
A.J. Aul, P.R. Fischer, J.S. O'Grady, K.C. Mara, J.A. Maxson, A.M. Meek, T.M. Petterson, T.D. Thacher, Population-based incidence of potentially life-threatening complications of hypocalcemia and the role of vitamin D deficiency. J. Pediatr. 211(98–104), e104 (2019). https://doi.org/10.1016/j.jpeds.2019.02.018
G.T. Williams, M. Brown, Laryngospasm in hypoparathyroidism. J. Laryngol. Otol. 88(4), 369–373 (1974). https://doi.org/10.1017/s0022215100078804
C.T. Chou, B. Siegel, D. Mehta, Stridor and apnea as the initial presentation of primary hypoparathyroidism. Int. J. Pediatr. Otorhinolaryngol. 80, 30–32 (2016). https://doi.org/10.1016/j.ijporl.2015.11.023
N.E. Cusano, J.P. Bilezikian, Signs and symptoms of hypoparathyroidism. Endocrinol. Metab. Clin. North Am. 47(4), 759–770 (2018). https://doi.org/10.1016/j.ecl.2018.07.001
R. Fjaer, E. Brodtkorb, A.M. Oye, Y. Sheng, M.D. Vigeland, K.A. Kvistad, P.H. Backe, K.K. Selmer, Generalized epilepsy in a family with basal ganglia calcifications and mutations in SLC20A2 and CHRNB2. Eur. J. Med. Genet. 58(11), 624–628 (2015). https://doi.org/10.1016/j.ejmg.2015.10.005
S.K. Bhadada, A. Bhansali, V. Upreti, S. Subbiah, N. Khandelwal, Spectrum of neurological manifestations of idiopathic hypoparathyroidism and pseudohypoparathyroidism. Neurol. India 59(4), 586–589 (2011). https://doi.org/10.4103/0028-3886.84342
L.S. Basser, F.C. Neale, A.W. Ireland, S. Posen, Epilepsy and electroencephalographic abnormalities in chronic surgical hypoparathyroidism. Ann. Intern Med 71(3), 507–515 (1969). https://doi.org/10.7326/0003-4819-71-3-507
A. Maiti, S. Chatterjee, Neuropsychiatric manifestations and their outcomes in chronic hypocalcaemia. J. Indian Med Assoc. 111(3), 174–177 (2013)
S.I. Rosenfeld, J.C. Bobrow, et al. Lens and cataracts. In: T.J. Leisenberg, G.L. Skuta, L.B. Couter (eds), Basic and Clinical Science Course. American Academy of Opthamology, Section 1, 45–69 (2004)
L. Underbjerg, T. Sikjaer, L. Rejnmark, Long-term complications in patients with hypoparathyroidism evaluated by biochemical findings: a case-control study. J. Bone Miner. Res. 33(5), 822–831 (2018). https://doi.org/10.1002/jbmr.3368
G. Tabacco, A.M. Naciu, D. Maggi, A. Santonati, C. Pedone, R. Cesareo, D. Bosco, G. Gaspa, N. Napoli, P. Pozzilli, S. Manfrini, A. Palermo, Cardiovascular autonomic neuropathy as a new complication of postsurgical chronic hypoparathyroidism. J. Bone Min. Res 34(3), 475–481 (2019). https://doi.org/10.1002/jbmr.3623
S. Velayuthan, N. Gungor, R. McVie, Hypocalcemic cardiomyopathy as initial presentation of primary hypoparathyroidism. Pediatrics Int. 56(4), e23–25 (2014). https://doi.org/10.1111/ped.12378
A. Avsar, A. Dogan, T. Tavli, A rare cause of reversible dilated cardiomyopathy: hypocalcemia. Echocardiography 21(7), 609–612 (2004). https://doi.org/10.1111/j.0742-2822.2004.03149.x
D.B. Newman, S.S. Fidahussein, D.T. Kashiwagi, K.A. Kennel, K.B. Kashani, Z. Wang, O. Altayar, M.H. Murad, Reversible cardiac dysfunction associated with hypocalcemia: a systematic review and meta-analysis of individual patient data. Heart Fail. Rev. 19(2), 199–205 (2014). https://doi.org/10.1007/s10741-013-9371-1
G. Tabacco, Y.D. Tay, N.E. Cusano, J. Williams, B. Omeragic, R. Majeed, M.G. Almonte, M.R. Rubin, J.P. Bilezikian, Quality of life in hypoparathyroidism improves with rhPTH(1-84) throughout 8 years of therapy. J. Clin. Endocrinol. Metab. 104(7), 2748–2756 (2019). https://doi.org/10.1210/jc.2018-02430
KDIGO, KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int. 7(Suppl.), 1–59 (2017).
P.C. Pham, P.A. Pham, S.V. Pham, P.T. Pham, P.M. Pham, P.T. Pham, Hypomagnesemia: a clinical perspective. Int. J. Nephrol. Renovascular Dis. 7, 219–230 (2014). https://doi.org/10.2147/IJNRD.S42054
E. Tsourdi, B. Langdahl, M. Cohen-Solal, B. Aubry-Rozier, E.F. Eriksen, N. Guanabens, B. Obermayer-Pietsch, S.H. Ralston, R. Eastell, M.C. Zillikens, Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 105, 11–17 (2017). https://doi.org/10.1016/j.bone.2017.08.003
J. Everts-Graber, S. Reichenbach, H.R. Ziswiler, U. Studer, T. Lehmann, A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J. Bone Min. Res. (2020). https://doi.org/10.1002/jbmr.3962
V. Kreutle, C. Blum, C. Meier, M. Past, B. Muller, P. Schutz, K. Borm, Bisphosphonate induced hypocalcaemia - report of six cases and review of the literature. Swiss Med. Wkly. 144, w13979 (2014). https://doi.org/10.4414/smw.2014.13979
C. Thongprayoon, P. Acharya, N.R. Aeddula, A. Torres-Ortiz, T. Bathini, K. Sharma, P. Ungprasert, K. Watthanasuntorn, M.L.G. Suarez, S.A. Salim, W. Kaewput, J. Chenbhanich, M.A. Mao, W. Cheungpasitporn, Effects of denosumab on bone metabolism and bone mineral density in kidney transplant patients: a systematic review and meta-analysis. Arch. Osteoporos. 14(1), 35 (2019). https://doi.org/10.1007/s11657-019-0587-0
D.M. McDonald-McGinn, K.E. Sullivan, B. Marino, N. Philip, A. Swillen, J.A. Vorstman, E.H. Zackai, B.S. Emanuel, J.R. Vermeesch, B.E. Morrow, P.J. Scambler, A.S. Bassett, 22q11.2 deletion syndrome. Nature reviews. Dis. Prim. 1, 15071 (2015). https://doi.org/10.1038/nrdp.2015.71
A. Linglart, M.A. Levine, H. Juppner, Pseudohypoparathyroidism. Endocrinol. Metab. Clin. North Am. 47(4), 865–888 (2018). https://doi.org/10.1016/j.ecl.2018.07.011
P.J. Malloy, D. Feldman, Genetic disorders and defects in vitamin d action. Endocrinol. Metab. Clin. North Am. 39(2), 333–346 (2010). https://doi.org/10.1016/j.ecl.2010.02.004.
P.J. Malloy, D. Feldman, Genetic disorders and defects in vitamin D action. Rheum. Dis. Clin. North Am. 38(1), 93–106 (2012). https://doi.org/10.1016/j.rdc.2012.03.009
J. Fong, A. Khan, Hypocalcemia: updates in diagnosis and management for primary care. Can. Fam. Phys. Med. de. famille canadien 58(2), 158–162 (2012)
Hammond, D.A., Stojakovic, J., Kathe, N., Tran, J., Clem, O.A., Erbach, K., King, J.: Effectiveness and Safety of Magnesium Replacement in Critically Ill Patients Admitted to the Medical Intensive Care Unit in an Academic Medical Center: A Retrospective, Cohort Study. J. Inten. Care Med. 885066617720631 (2017). https://doi.org/10.1177/0885066617720631
S. Minisola, J. Pepe, P. Donato, E. Vigna, M. Occhiuto, F. Ferrone, F. Biamonte, V. Cecchetti, V.C. Danese, C. Sonato, B.I. P, L. Colangelo, C. Cipriani, Replenishment of vitamin D status: theoretical and practical considerations. Hormones 18(1), 3–5 (2019). https://doi.org/10.1007/s42000-018-0040-6
W.T. Clusin, Calcium and cardiac arrhythmias: DADs, EADs, and alternans. Crit. Rev. Clin. Lab. Sci. 40(3), 337–375 (2003). https://doi.org/10.1080/713609356
A. Sanabria, A. Rojas, J. Arevalo, Meta-analysis of routine calcium/vitamin D3 supplementation versus serum calcium level-based strategy to prevent postoperative hypocalcaemia after thyroidectomy. Br. J. Surg. 106(9), 1126–1137 (2019). https://doi.org/10.1002/bjs.11216
O. Edafe, C.E. Mech, S.P. Balasubramanian, Calcium, vitamin D or recombinant parathyroid hormone for managing post-thyroidectomy hypoparathyroidism. Cochrane Database Syst. Rev. 5, CD012845 (2019). https://doi.org/10.1002/14651858.CD012845.pub2
J. Bollerslev, L. Rejnmark, C. Marcocci, D.M. Shoback, A. Sitges-Serra, W. van Biesen, O.M. Dekkers, European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. Eur. J. Endocrinol. 173(2), G1–20 (2015). https://doi.org/10.1530/EJE-15-0628
M.L. Brandi, J.P. Bilezikian, D. Shoback, R. Bouillon, B.L. Clarke, R.V. Thakker, A.A. Khan, J.T. Potts Jr, Management of hypoparathyroidism: summary statement and guidelines. J. Clin. Endocrinol. Metab. 101(6), 2273–2283 (2016). https://doi.org/10.1210/jc.2015-3907
L.A. Orloff, S.M. Wiseman, V.J. Bernet, T.J. Fahey III, A.R. Shaha, M.L. Shindo, S.K. Snyder, B.C. Stack Jr, J.B. Sunwoo, M.B: Wang, American thyroid association staement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid. 28(7), 830–841 (2018). https://doi.org/10.1089/thy.2017.0309
E. Coudenys, T. Van Meerhaeghe, D. Unuane, R. Buyl, B. Bravenboer, Long-term treatment with calcitriol in postsurgical hypoparathyroidism leads to renal function decline. Horm. Metab. Res. 51(6), 362–366 (2019). https://doi.org/10.1055/a-0902-8476
M. Mannstadt, B.L. Clarke, T. Vokes, M.L. Brandi, L. Ranganath, W.D. Fraser, P. Lakatos, L. Bajnok, R. Garceau, L. Mosekilde, H. Lagast, D. Shoback, J.P. Bilezikian, Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 1(4), 275–283 (2013). https://doi.org/10.1016/S2213-8587(13)70106-2
K.K. Winer, C.W. Ko, J.C. Reynolds, K. Dowdy, M. Keil, D. Peterson, L.H. Gerber, C. McGarvey, G.B. Cutler Jr, Long-term treatment of hypoparathyroidism: a randomized controlled study comparing parathyroid hormone-(1-34) versus calcitriol and calcium. J. Clin. Endocrinol. Metab. 88(9), 4214–4220 (2003). https://doi.org/10.1210/jc.2002-021736
K.K. Winer, N. Sinaii, J. Reynolds, D. Peterson, K. Dowdy, G.B. Cutler Jr, Long-term treatment of 12 children with chronic hypoparathyroidism: a randomized trial comparing synthetic human parathyroid hormone 1-34 versus calcitriol and calcium. J. Clin. Endocrinol. Metab. 95(6), 2680–2688 (2010). https://doi.org/10.1210/jc.2009-2464
A. Palermo, A. Santonati, G. Tabacco, D. Bosco, A. Spada, C. Pedone, B. Raggiunti, T. Doris, D. Maggi, F. Grimaldi, S. Manfrini, F. Vescini, PTH(1-34) for surgical hypoparathyroidism: a 2-year prospective, open-label investigation of efficacy and quality of life. J. Clin. Endocrinol. Metab. 103(1), 271–280 (2018). https://doi.org/10.1210/jc.2017-01555
S. Minisola, C. Cipriani, G.D. Grotta, L. Colangelo, M. Occhiuto, P. Biondi, C. Sonato, E. Vigna, M. Cilli, J. Pepe, Update on the safety and efficacy of teriparatide in the treatment of osteoporosis. Therapeutic Adv. Musculoskelet. Dis. 11, 1759720X19877994 (2019). https://doi.org/10.1177/1759720X19877994
Y. Ramakrishnan, H.C. Cocks, Impact of recombinant PTH on management of hypoparathyroidism: a systematic review. Eur. Arch. Oto-Rhino-laryngol 273(4), 827–835 (2016). https://doi.org/10.1007/s00405-014-3484-6
Tuli, G., Buganza, R., Tessaris, D., Einaudi, S., Matarazzo, P., de Sanctis, L.: Teriparatide (rhPTH 1-34) treatment in the pediatric age: long-term efficacy and safety data in a cohort with genetic hypoparathyroidism. Endocrine (2019). https://doi.org/10.1007/s12020-019-02128-z
R.I. Gafni, L.C. Guthrie, M.H. Kelly, B.A. Brillante, C.M. Christie, J.C. Reynolds, N.A. Yovetich, R. James, M.T. Collins, Transient increased calcium and calcitriol requirements after discontinuation of human synthetic parathyroid hormone 1-34 (hPTH 1-34) replacement therapy in hypoparathyroidism. J. Bone Miner. Res. 30(11), 2112–2118 (2015). https://doi.org/10.1002/jbmr.2555
Y.D. Tay, G. Tabacco, N.E. Cusano, J. Williams, B. Omeragic, R. Majeed, M. Gomez Almonte, J.P. Bilezikian, M.R. Rubin, Therapy of hypoparathyroidism with rhPTH(1-84): a prospective, 8-year investigation of efficacy and safety. J. Clin. Endocrinol. Metab. 104(11), 5601–5610 (2019). https://doi.org/10.1210/jc.2019-00893
R.I. Gafni, C.B. Langman, L.C. Guthrie, B.A. Brillante, R. James, N.A. Yovetich, A.M. Boyce, M.T. Collins, Hypocitraturia is an untoward side effect of synthetic human parathyroid hormone (hpth) 1-34 therapy in hypoparathyroidism that may increase renal morbidity. J. Bone Min. Res 33(10), 1741–1747 (2018). https://doi.org/10.1002/jbmr.3480
K.K. Winer, B. Zhang, J.A. Shrader, D. Peterson, M. Smith, P.S. Albert, G.B. Cutler Jr, Synthetic human parathyroid hormone 1-34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J. Clin. Endocrinol. Metab. 97(2), 391–399 (2012). https://doi.org/10.1210/jc.2011-1908
L. Holten-Andersen, S. Pihl, C.E. Rasmussen, J. Zettler, G. Maitro, J. Baron, S. Heinig, E. Hoffmann, T. Wegge, M. Krusch, F. Faltinger, S. Killian, K. Sprogoe, D.B. Karpf, V.M. Breinholt, F. Cleemann, Design and preclinical development of transcon pth, an investigational sustained-release pth replacement therapy for hypoparathyroidism. J. Bone Miner. Res.: Off. J. Am. Soc. Bone Miner. Res. 34(11), 2075–2086 (2019). https://doi.org/10.1002/jbmr.3824
G. Marcucci, M.L. Brandi, A new era for chronic management of hypoparathyroidism: parathyroid hormone peptides. Front. Horm. Res. 51, 165–171 (2019). https://doi.org/10.1159/000491047
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.M. served as speaker for Abiogen, Amgen, Bruno Farmaceutici, Diasorin, Eli Lilly, Shire, Sandoz, Takeda. He also served in advisory board of Abiogen, Kyowa Kirin, Pfizer, UCB. All other authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pepe, J., Colangelo, L., Biamonte, F. et al. Diagnosis and management of hypocalcemia. Endocrine 69, 485–495 (2020). https://doi.org/10.1007/s12020-020-02324-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02324-2