Abstract
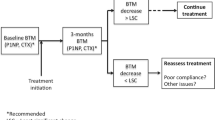
The purpose of the present study was to examine of the current role of bone turnover markers (BTMs) in the management of osteoporosis. Perusal of the literature examines the available evidence for the utility of BTMs for decision to treat and for the monitoring of treatment for osteoporosis. There is no evidence for the use of BTMs for fracture risk calculation, decision to treat or for treatment selection. A very abnormal BTM value may be a clue to the presence of bone pathology other than uncomplicated osteoporosis. Whilst changes to BTMs following various osteoporosis treatments are well defined, their utility in monitoring individual patients has been less well established. Some fracture outcome-based data exist for the use of u-NTX target of <21 nmol BCE/mmol for antiresorptive therapy; the equivalent s-CTX level is ~250 ng/L. Suboptimal BTM response to treatment may indicate non-compliance or the presence of secondary causes of osteoporosis which may need addressing. Studies are needed to establish treatment targets based on fracture outcomes for commonly used BTMs for each established osteoporosis therapy.
Similar content being viewed by others
References
P.D. Delmas, R. Eastell, P. Garnero, M.J. Seibel, J. Stepan, The use of biochemical markers of bone turnover in osteoporosis. Osteoporos. Int. 11, 2–17 (2000)
T. Cundy, I.R. Reid, Paget’s disease of bone. Clin. Biochem. 45(1–2), 43–48 (2012)
S. Vasikaran, R. Eastell, O. Bruyère, A.J. Foldes, P. Garnero, A. Griesmacher, M. McClung, H.A. Morris, S. Silverman, T. Trenti, D.A. Wahl, C. Cooper, J.A. Kanis, IOF-IFCC Bone Marker Standards Working Group, Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos. Int. 22, 391–420 (2011)
D. Bauer, J. Krege, N. Lane, E. Leary, C. Libanati, P. Miller, G. Myers, S. Silverman, H.W. Vesper, D. Lee, M. Payette, S. Randall, National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos. Int. 23, 2425–2433 (2012)
P. Garnero, E. Sornay-Rendu, B. Claustra, P.D. Delmas, Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J. Bone. Miner. Res. 15(8), 1526–1536 (2000)
P. Garnero, E. Hausherr, M.C. Chapuy, C. Marcelli, H. Grandjean, C. Muller, C. Cormier, G. Bréart, P.J. Meunier, P.D. Delmas, Markers of bone resorption predict hip fracture in elderly women: the EPIDOS prospective study. J. Bone Miner. Res. 11(10), 1531–1538 (1996)
H. Johansson, A. Odén, J.A. Kanis, E.V. McCloskey, H.A. Morris, C. Cooper, S. Vasikaran, IFCC-IOF Joint Working Group on Standardisation of Biochemical Markers of Bone Turnover, A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif. Tissue Int. 94(5), 560–567 (2014)
J.A. Kanis, O. Johnell, A. Oden, H. Johansson, E. McCloskey, FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. 19, 385–397 (2008)
I. Baxter, A. Rogers, R. Eastell, N. Peel, Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos. Int. 24(3), 941–947 (2013)
D.C. Bauer, P. Garnero, M.C. Hochberg, A. Santora, P. Delmas, S.K. Ewing, D.M. Black, Fracture Intervention Research Group, Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J. Bone Miner. Res. 21(2), 292–299 (2006)
P.D. Delmas, A.A. Licata, J.Y. Reginster, G.G. Crans, P. Chen, D.A. Misurski, R.B. Wagman, B.H. Mitlak, Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone 39(2), 237–243 (2006)
J.A. Clowes, N.F. Peel, R. Eastell, The impact of monitoring on adherence and persistence with antiresorptive treatment for Osteoporos Int postmenopausal osteoporosis: a randomized controlled trial. J. Clin. Endocrinol. Metab. 89, 1117–1123 (2004)
P.D. Delmas, B. Vrijens, R. Eastell, C. Roux, H.A. Pols, J.D. Ringe, A. Grauer, D. Cahall, N.B. Watts, Improving Measurements of Persistence on Actonel Treatment (IMPACT) Investigators, Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 92, 1296–1304 (2007)
R. Eastell, I. Barton, R.A. Hannon, A. Chines, P. Garnero, P.D. Delmas, Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J. Bone Miner. Res. 18, 1051–1056 (2003)
D.C. Bauer, D.M. Black, P. Garnero, M. Hochberg, S. Ott, J. Orloff, D.E. Thompson, S.K. Ewing, P.D. Delmas, Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J. Bone Miner. Res. 19, 1250–1258 (2004)
P.D. Delmas, F. Munoz, D.M. Black, F. Cosman, S. Boonen, N.B. Watts, D. Kendler, E.F. Eriksen, P.G. Mesenbrink, R. Eastell, The HORIZON-PFT Research Group, Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. J. Bone Miner. Res. 24, 1544–1551 (2009)
P. Chen, J.H. Satterwhite, A.A. Licata, E.M. Lewiecki, A.A. Sipos, D.M. Misurski, R.B. Wagman, Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J. Bone Miner. Res. 20, 962–970 (2005)
E. Fink, C. Cormier, P. Steinmetz, C. Kindermans, Y. Le Bouc, J.C. Souberbielle, Differences in the capacity of several biochemical bone markers to assess high bone turnover in early menopause and response to alendronate therapy. Osteoporos. Int. 1, 295–303 (2000)
P. Bergmann, J.J. Body, S. Boonen, Y. Boutsen, J.P. Devogelaer, S. Goemaere, J.M. Kaufman, J.Y. Reginster, V. Gangji, Members of the Advisory Board on Bone Markers, Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int. J. Clin. Pract. 63, 19–26 (2009)
R. Eastell, P. Garnero, C. Audebert, D. Cahall, Reference intervals of bone turnover markers in healthy premenopausal women: results from a cross-sectional European study. Bone 50(5), 1141–1147 (2012)
S.A. Chubb, C.D. Mandelt, S.D. Vasikaran, Comparison of results from commercial assays for plasma CTX: the need for harmonization. Clin. Biochem. 48, 519–524 (2015)
D.A. Eekman, I.E. Bultink, A.C. Heijboer, B.A. Dijkmans, W.F. Lems, Bone turnover is adequately suppressed in osteoporotic patients treated with bisphosphonates in daily practice. BMC Musculoskelet. Disord. 12, 167 (2001)
R. Eastell, B. Vrijens, D.L. Cahall, J.D. Ringe, P. Garnero, N.B. Watts, Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J. Bone Miner. Res. 26(7), 1662–1669 (2011)
S.A. Chubb, C. Mandelt, S. Vasikaran, Comparison of clinical cut-points and treatment targets for urine NTX and plasma βCTX-I in osteoporosis. Clin. Biochem. (2015). doi:10.1016/j.clinbiochem.2015.12.002
A.I. Sebba, S.L. Bonnick, R. Kagan, D.E. Thompson, C.S. Skalky, E. Chen, A.E. de Papp, Fosamax Actonel Comparison Trial Investigators, Response to therapy with once-weekly alendronate 70 mg compared to once-weekly risedronate 35 mg in the treatment of postmenopausal osteoporosis. Curr. Med. Res. Opin. 20(12), 2031–2041 (2004)
K. Saag, R. Lindsay, A. Kriegman, E. Beamer, W. Zhou, A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone 40(5), 1238–1243 (2007)
D.L. Kendler, C. Roux, C.L. Benhamou, J.P. Brown, M. Lillestol, S. Siddhanti, H.S. Man, J. San Martin, H.G. Bone, Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J. Bone. Miner. Res. 25, 72–78 (2010)
R. Eastell, J.H. Krege, P. Chen, E.V. Glass, J.Y. Reginster, Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr. Med. Res. Opin. 22, 61–66 (2006)
M. Tsujimoto, P. Chen, A. Miyauchi, H. Sowa, J.H. Krege, PINP as an aid for monitoring patients treated with teriparatide. Bone 48, 798–803 (2009)
D.C. Bauer, A. Schwartz, L. Palermo, J. Cauley, M. Hochberg, A. Santora, S.R. Cummings, D.M. Black, Fracture prediction after discontinuation of 4 to 5 years of alendronate therapy: the FLEX study. JAMA Intern. Med. 174(7), 1126–1134 (2014)
R.E. Marx, J.E. Cill Jr, J.J. Ulloa, Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral Maxillofac. Surg. 65(12), 2397–2410 (2007)
S.L. Ruggiero, T.B. Dodson, J. Fantasia, R. Goodday, T. Aghaloo, B. Mehrotra, F. O’Ryan, American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J. Oral Maxillofac. Surg. 72(10), 1938–1956 (2014)
A. Hutcheson, A. Cheng, R. Kunchar, B. Stein, P. Sambrook, A. Goss, A C-terminal crosslinking telopeptide test-based protocol for patients on oral bisphosphonates requiring extraction: a prospective single-center controlled study. J. Oral Maxillofac. Surg. 72(8), 1456–1462 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vasikaran, S.D., Paul Chubb, S.A. The use of biochemical markers of bone turnover in the clinical management of primary and secondary osteoporosis. Endocrine 52, 222–225 (2016). https://doi.org/10.1007/s12020-016-0900-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0900-2