Abstract
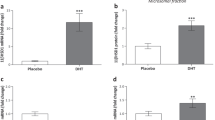
Polycystic ovary syndrome (PCOS) is associated with an altered plasma lipid profile and increased risk for cardiovascular diseases. We hypothesized that molecular mechanisms underlying cardiac pathology in PCOS involve changes in expression and subcellular localization of several key proteins involved in cardiac lipid transport and metabolism, such as fatty acid transporter CD36, lipin 1, peroxisome proliferator-activated receptor α (PPARα), peroxisome proliferator-activated receptor γ coactivator-1 (PGC1), and carnitine palmitoyltransferase 1 (CPT1). We used the animal model of PCOS obtained by treating female rats with dihydrotestosterone (DHT). Protein levels of CD36, lipin 1, PPARα, PGC1, and antioxidative enzymes were assessed by Western blot in different cardiac cell compartments. Cardiac triglycerides (TG) and lipid peroxidation were also measured. The content of CD36 was decreased in both the cardiac plasma membranes and intracellular pool. On the other hand, total content of cardiac lipin 1 in DHT-treated rats was elevated, in contrast to decreased microsomal lipin 1 content. An increase in nuclear content of lipin 1 was observed together with elevation of nuclear PPARα and PGC1, and an increase in CPT1 expression. However, lipid peroxidation was reduced in the heart, without alterations in antioxidative enzymes expression and cardiac TG content. The results indicate that treatment of female rats with DHT is accompanied by a decrease of fatty acid uptake and a reduction of lipid peroxidation in the heart. The observed elevation of lipin 1, PPARα, PGC1, and CPT1 expression suggests that cardiac fatty acid metabolism is shifted toward mitochondrial beta oxidation.




Similar content being viewed by others
References
G. Garruti, R. Depalo, M.G. Vita, F. Lorusso, F. Giampetruzzi, A.B. Damato, F. Giorgino, Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod. Biomed. Online 19(4), 552–563 (2009)
H. Teede, A. Deeks, L. Moran, Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 8, 41 (2010). doi:10.1186/1741-7015-8-41
R.A. Wild, E. Carmina, E. Diamanti-Kandarakis, A. Dokras, H.F. Escobar-Morreale, W. Futterweit, R. Lobo, R.J. Norman, E. Talbott, D.A. Dumesic, Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J. Clin. Endocrinol. Metab. 95(5), 2038–2049 (2010). doi:10.1210/jc.2009-2724
E. Talbott, A. Clerici, S.L. Berga, L. Kuller, D. Guzick, K. Detre, T. Daniels, R.A. Engberg, Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J. Clin. Epidemiol. 51(5), 415–422 (1998)
R.A. Wild, Dyslipidemia in PCOS. Steroids 77(4), 295–299 (2012). doi:10.1016/j.steroids.2011.12.002
A. Baranova, T.P. Tran, A. Birerdinc, Z.M. Younossi, Systematic review: association of polycystic ovary syndrome with metabolic syndrome and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 33(7), 801–814 (2011). doi:10.1111/j.1365-2036.2011.04579.x
D. Macut, D. Panidis, B. Glisic, N. Spanos, M. Petakov, J. Bjekic, O. Stanojlovic, D. Rousso, A. Kourtis, I. Bozic, S. Damjanovic, Lipid and lipoprotein profile in women with polycystic ovary syndrome. Can. J. Physiol. Pharmacol. 86(4), 199–204 (2008). doi:10.1139/Y08-014
H.E. Westerveld, M. Hoogendoorn, A.W. de Jong, A.J. Goverde, B.C. Fauser, G.M. Dallinga-Thie, Cardiometabolic abnormalities in the polycystic ovary syndrome: pharmacotherapeutic insights. Pharmacol. Ther. 119(3), 223–241 (2008). doi:10.1016/j.pharmthera.2008.04.009
I.F. Kodde, J. van der Stok, R.T. Smolenski, J.W. de Jong, Metabolic and genetic regulation of cardiac energy substrate preference. Comp. Biochem. Physiol. 146(1), 26–39 (2007). doi:10.1016/j.cbpa.2006.09.014
J.J. Luiken, S.L. Coort, D.P. Koonen, D.J. van der Horst, A. Bonen, A. Zorzano, J.F. Glatz, Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch. 448(1), 1–15 (2004). doi:10.1007/s00424-003-1199-4
J.F. Glatz, A. Bonen, D.M. Ouwens, J.J. Luiken, Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc. Drugs Ther. 20(6), 471–476 (2006). doi:10.1007/s10557-006-0582-8
J.G. Nickerson, I. Momken, C.R. Benton, J. Lally, G.P. Holloway, X.X. Han, J.F. Glatz, A. Chabowski, J.J. Luiken, A. Bonen, Protein-mediated fatty acid uptake: regulation by contraction, AMP-activated protein kinase, and endocrine signals. Appl. Physiol. Nutr. Metab. 32(5), 865–873 (2007). doi:10.1139/H07-084
D.N. Brindley, B.P. Kok, P.C. Kienesberger, R. Lehner, J.R. Dyck, Shedding light on the enigma of myocardial lipotoxicity: the involvement of known and putative regulators of fatty acid storage and mobilization. Am. J. Physiol. Endocrinol. Metab. 298(5), E897–E908 (2010). doi:10.1152/ajpendo.00509.2009
J.J. Luiken, D.P. Koonen, J. Willems, A. Zorzano, C. Becker, Y. Fischer, N.N. Tandon, G.J. Van Der Vusse, A. Bonen, J.F. Glatz, Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 51(10), 3113–3119 (2002)
S.L. Coort, A. Bonen, G.J. van der Vusse, J.F. Glatz, J.J. Luiken, Cardiac substrate uptake and metabolism in obesity and type-2 diabetes: role of sarcolemmal substrate transporters. Mol. Cell. Biochem. 299(1–2), 5–18 (2007). doi:10.1007/s11010-005-9030-5
K. Reue, D.N. Brindley, Thematic review series: glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 49(12), 2493–2503 (2008). doi:10.1194/jlr.R800019-JLR200
C. Burgdorf, L. Hansel, M. Heidbreder, O. Johren, F. Schutte, H. Schunkert, T. Kurz, Suppression of cardiac phosphatidate phosphohydrolase 1 activity and lipin mRNA expression in Zucker diabetic fatty rats and humans with type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 390(1), 165–170 (2009). doi:10.1016/j.bbrc.2009.09.108
M.S. Mitra, J.D. Schilling, X. Wang, P.Y. Jay, J.M. Huss, X. Su, B.N. Finck, Cardiac lipin 1 expression is regulated by the peroxisome proliferator activated receptor gamma coactivator 1α/estrogen related receptor axis. J. Mol. Cell. Cardiol. 51(1), 120–128 (2011). doi:10.1016/j.yjmcc.2011.04.009
B.P. Kok, P.C. Kienesberger, J.R. Dyck, D.N. Brindley, Relationship of glucose and oleate metabolism to cardiac function in lipin-1 deficient (fld) mice. J. Lipid Res. 53(1), 105–118 (2012). doi:10.1194/jlr.M019430
T.E. Harris, B.N. Finck, Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol. Metab. 22(6), 226–233 (2011). doi:10.1016/j.tem.2011.02.006
D. Shi, D.F. Vine, Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil. Steril. 98(1), 185–193 (2012). doi:10.1016/j.fertnstert.2012.04.006
L. Manneras, S. Cajander, A. Holmang, Z. Seleskovic, T. Lystig, M. Lonn, E. Stener-Victorin, A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 148(8), 3781–3791 (2007). doi:10.1210/en.2007-0168
D.M. Ouwens, M. Diamant, M. Fodor, D.D. Habets, M.M. Pelsers, M. El Hasnaoui, Z.C. Dang, C.E. van den Brom, R. Vlasblom, A. Rietdijk, C. Boer, S.L. Coort, J.F. Glatz, J.J. Luiken, Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 50(9), 1938–1948 (2007). doi:10.1007/s00125-007-0735-8
E.L. van Houten, P. Kramer, A. McLuskey, B. Karels, A.P. Themmen, J.A. Visser, Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology 153(6), 2861–2869 (2012). doi:10.1210/en.2011-1754
A.S. Caldwell, L.J. Middleton, M. Jimenez, R. Desai, A.C. McMahon, C.M. Allan, D.J. Handelsman, K.A. Walters, Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 155(8), 3146–3159 (2014). doi:10.1210/en.2014-1196
S. Tepavcevic, D.V. Milutinovic, D. Macut, Z. Zakula, M. Nikolic, I. Bozic-Antic, S. Romic, J. Bjekic-Macut, G. Matic, G. Koricanac, Dihydrotestosterone deteriorates cardiac insulin signaling and glucose transport in the rat model of polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 142, 71–76 (2014). doi:10.1016/j.jsbmb.2014.01.006
E.T. Wang, I.A. Ku, S.J. Shah, M.L. Daviglus, P.J. Schreiner, S.H. Konety, O.D. Williams, D. Siscovick, K. Bibbins-Domingo, Polycystic ovary syndrome is associated with higher left ventricular mass index: the CARDIA women’s study. J Clin. Endocrinol. Metab. 97(12), 4656–4662 (2012). doi:10.1210/jc.2012-1597
F. Orio Jr, S. Palomba, L. Spinelli, T. Cascella, L. Tauchmanova, F. Zullo, G. Lombardi, A. Colao, The cardiovascular risk of young women with polycystic ovary syndrome: an observational, analytical, prospective case-control study. J. Clin. Endocrinol. Metab. 89(8), 3696–3701 (2004). doi:10.1210/jc.2003-032049
B.G. Stuckey, N. Opie, A.J. Cussons, G.F. Watts, V. Burke, Clustering of metabolic and cardiovascular risk factors in the polycystic ovary syndrome: a principal component analysis. Metabolism 63(8), 1071–1077 (2014). doi:10.1016/j.metabol.2014.05.004
M. Fassnacht, N. Schlenz, S.B. Schneider, S.A. Wudy, B. Allolio, W. Arlt, Beyond adrenal and ovarian androgen generation: increased peripheral 5 alpha-reductase activity in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 88(6), 2760–2766 (2003)
M.E. Silfen, M.R. Denburg, A.M. Manibo, R.A. Lobo, R. Jaffe, M. Ferin, L.S. Levine, S.E. Oberfield, Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J. Clin. Endocrinol. Metab. 88(10), 4682–4688 (2003)
J. Folch, M. Lees, G.H. Sloane-Stanley, A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226(1), 497–509 (1957)
G. Koricanac, S. Tepavcevic, S. Romic, M. Zivkovic, M. Stojiljkovic, T. Milosavljevic, A. Stankovic, M. Petkovic, T. Kamceva, Z. Zakula, Estradiol enhances effects of fructose rich diet on cardiac fatty acid transporter CD36 and triglycerides accumulation. Eur. J. Pharmacol. 694(1–3), 127–134 (2012). doi:10.1016/j.ejphar.2012.08.007
M.J. Fletcher, A colorimetric method for estimating serum triglycerides. Clin. Chim. Acta 22(3), 393–397 (1968)
S. Romic, S. Tepavcevic, Z. Zakula, T. Milosavljevic, M. Kostic, M. Petkovic, G. Koricanac, Gender differences in the expression and cellular localization of lipin 1 in the hearts of fructose-fed rats. Lipids (2014). doi:10.1007/s11745-014-3909-4
U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259), 680–685 (1970)
S. Tepavcevic, G. Koricanac, Z. Zakula, T. Milosavljevic, M. Stojiljkovic, E.R. Isenovic, Interaction between insulin and estradiol in regulation of cardiac glucose and free fatty acid transporters. Horm. Metab. Res. 43(8), 524–530 (2011). doi:10.1055/s-0031-1280784
R.W. Evans, A.P. Farwell, L.E. Braverman, Nuclear thyroid hormone receptor in the rat uterus. Endocrinology 113(4), 1459–1463 (1983)
O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall, Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193(1), 265–275 (1951)
J.A. Buege, S.D. Aust, Microsomal lipid peroxidation. Methods Enzymol. 52, 302–310 (1978)
I. Lambrinoudaki, Cardiovascular risk in postmenopausal women with the polycystic ovary syndrome. Maturitas 68(1), 13–16 (2011). doi:10.1016/j.maturitas.2010.09.005
V. Skov, D. Glintborg, S. Knudsen, T. Jensen, T.A. Kruse, Q. Tan, K. Brusgaard, H. Beck-Nielsen, K. Hojlund, Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes 56(9), 2349–2355 (2007). doi:10.2337/db07-0275
K.M. Seow, Y.L. Tsai, J.L. Hwang, W.Y. Hsu, L.T. Ho, C.C. Juan, Omental adipose tissue overexpression of fatty acid transporter CD36 and decreased expression of hormone-sensitive lipase in insulin-resistant women with polycystic ovary syndrome. Hum. Reprod. 24(8), 1982–1988 (2009). doi:10.1093/humrep/dep122
B. Mlinar, M. Pfeifer, E. Vrtacnik-Bokal, M. Jensterle, J. Marc, Decreased lipin 1 beta expression in visceral adipose tissue is associated with insulin resistance in polycystic ovary syndrome. Eur. J. Endocrinol. 159(6), 833–839 (2008). doi:10.1530/EJE-08-0387
R. Azziz, E. Carmina, D. Dewailly, E. Diamanti-Kandarakis, H.F. Escobar-Morreale, W. Futterweit, O.E. Janssen, R.S. Legro, R.J. Norman, A.E. Taylor, S.F. Witchel, The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil. Steril. 91(2), 456–488 (2009). doi:10.1016/j.fertnstert.2008.06.035
M. Nikolic, D. Macut, A. Djordjevic, N. Velickovic, N. Nestorovic, B. Bursac, I.B. Antic, J.B. Macut, G. Matic, D.V. Milutinovic, Possible involvement of glucocorticoids in 5α-dihydrotestosterone-induced PCOS-like metabolic disturbances in the rat visceral adipose tissue. Mol. Cell. Endocrinol. 399, 22–31 (2015). doi:10.1016/j.mce.2014.08.013
J. Smith, X. Su, R. El-Maghrabi, P.D. Stahl, N.A. Abumrad, Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J. Biol. Chem. 283(20), 13578–13585 (2008). doi:10.1074/jbc.M800008200
B.P. Kok, J.R. Dyck, T.E. Harris, D.N. Brindley, Differential regulation of the expressions of the PGC-1α splice variants, lipins, and PPARα in heart compared to liver. J. Lipid Res. 54(6), 1662–1677 (2013). doi:10.1194/jlr.M036624
C.R. Benton, X.X. Han, M. Febbraio, T.E. Graham, A. Bonen, Inverse relationship between PGC-1α protein expression and triacylglycerol accumulation in rodent skeletal muscle. J. Appl. Physiol. 100(2), 377–383 (2006). doi:10.1152/japplphysiol.00781.2005
Q. Yang, Y. Li, Roles of PPARs on regulating myocardial energy and lipid homeostasis. J. Mol. Med. (Berl) 85(7), 697–706 (2007). doi:10.1007/s00109-007-0170-9
B.N. Finck, J.J. Lehman, T.C. Leone, M.J. Welch, M.J. Bennett, A. Kovacs, X. Han, R.W. Gross, R. Kozak, G.D. Lopaschuk, D.P. Kelly, The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Investig. 109(1), 121–130 (2002). doi:10.1172/JCI14080
J.G. Duncan, J.L. Fong, D.M. Medeiros, B.N. Finck, D.P. Kelly, Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation 115(7), 909–917 (2007). doi:10.1161/CIRCULATIONAHA.106.662296
G.D. Lopaschuk, J.R. Ussher, C.D. Folmes, J.S. Jaswal, W.C. Stanley, Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90(1), 207–258 (2010). doi:10.1152/physrev.00015.2009
A. Dikmen, A.M. Ergenoglu, A.O. Yeniel, O.Y. Dilsiz, G. Ercan, H. Yilmaz, Evaluation of glycemic and oxidative/antioxidative status in the estradiol valerate-induced PCOS model of rats. Eur. J. Obstet. Gynecol. Reprod. Biol. 160(1), 55–59 (2012). doi:10.1016/j.ejogrb.2011.09.042
D. Macut, T. Simic, A. Lissounov, M. Pljesa-Ercegovac, I. Bozic, T. Djukic, J. Bjekic-Macut, M. Matic, M. Petakov, S. Suvakov, S. Damjanovic, A. Savic-Radojevic, Insulin resistance in non-obese women with polycystic ovary syndrome: relation to byproducts of oxidative stress. Exp. Clin. Endocrinol. Diabetes 119(7), 451–455 (2011). doi:10.1055/s-0031-1279740
L. Ibarra-Lara, E. Hong, E. Soria-Castro, J.C. Torres-Narvaez, F. Perez-Severiano, L. Del Valle-Mondragon, L.G. Cervantes-Perez, M. Ramirez-Ortega, G.S. Pastelin-Hernandez, A. Sanchez-Mendoza, Clofibrate PPARα activation reduces oxidative stress and improves ultrastructure and ventricular hemodynamics in no-flow myocardial ischemia. J. Cardiovasc. Pharmacol. 60(4), 323–334 (2012). doi:10.1097/FJC.0b013e31826216ed
M. Baranowski, A. Blachnio-Zabielska, J. Gorski, Peroxisome proliferator-activated receptor alpha activation induces unfavourable changes in fatty acid composition of myocardial phospholipids. J. Physiol. Pharmacol. 60(2), 13–20 (2009)
Q. Tian, F.A. Grzemski, S. Panagiotopoulos, J.T. Ahokas, Peroxisome proliferator-activated receptor alpha agonist, clofibrate, has profound influence on myocardial fatty acid composition. Chem-Biol. Interact. 160(3), 241–251 (2006). doi:10.1016/j.cbi.2006.02.003
A. Ayala, M.F. Munoz, S. Arguelles, Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 360438 (2014). doi:10.1155/2014/360438
J. Keller, M. Mandala, P. Casson, G. Osol, Endothelial dysfunction in a rat model of PCOS: evidence of increased vasoconstrictor prostanoid activity. Endocrinology 152(12), 4927–4936 (2011). doi:10.1210/en.2011-1424
J. Langfort, S. Jagsz, P. Dobrzyn, Z. Brzezinska, B. Klapcinska, H. Galbo, J. Gorski, Testosterone affects hormone-sensitive lipase (HSL) activity and lipid metabolism in the left ventricle. Biochem. Biophys. Res. Commun. 399(4), 670–676 (2010). doi:10.1016/j.bbrc.2010.07.140
T.E. Harris, T.A. Huffman, A. Chi, J. Shabanowitz, D.F. Hunt, A. Kumar, J.C. Lawrence Jr, Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J. Biol. Chem. 282(1), 277–286 (2007). doi:10.1074/jbc.M609537200
R.W. Brownsey, A.N. Boone, M.F. Allard, Actions of insulin on the mammalian heart: metabolism, pathology and biochemical mechanisms. Cardiovasc. Res. 34(1), 3–24 (1997)
Acknowledgments
This study was supported by the Project Grant No. III41009, from the Ministry of Education, Science and Technological Development, Republic of Serbia. Authors are particularly thankful to Marija Takić, from the Laboratory for Nutrition and Metabolism of the Institute for Medical Research, University of Belgrade, Serbia, for her assistance in the measurement of cardiac triglycerides.
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
All animal procedures were in compliance with the EU Directive (2010/63/EU) on the protection of animals used for experimental and other scientific purposes, and were approved by the Ethical Committee for the Use of Laboratory Animals of the Institute for Biological Research “Siniša Stanković”, University of Belgrade (No. 2-20/10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tepavčević, S., Milutinović, D.V., Macut, D. et al. Cardiac fatty acid uptake and metabolism in the rat model of polycystic ovary syndrome. Endocrine 50, 193–201 (2015). https://doi.org/10.1007/s12020-015-0558-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0558-1