Abstract
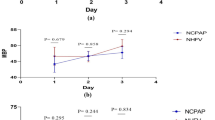
Children with congenital heart disease (CHD) have associated extracardiac co-morbidities at the time of surgery and during ongoing growth and development. Perioperative events include disrupted glucose homeostasis, capillary leak, and fluid retention. The hypothalamic–pituitary–adrenal (HPA) axis has an important role in homeostasis in that the secretion of cortisol contributes to the response to stress, glucose regulation, blood volume control, and immune regulation. We investigated the diurnal rhythm of the HPA axis in infants with CHD by measuring salivary cortisol in the morning (0600–0900 h—circadian peak) and evening (2100–2400 h—circadian nadir). Twenty-nine infants aged 12 weeks to 1 year were included: 16 with acyanotic disease (SpO2 ≥ 90 %) and 13 with cyanotic disease (SpO2 < 90 %). Morning salivary cortisol was similar between the two groups [acyanotic 7.0 nmol/L (1.8–23.1); cyanotic 9.7 nmol/L (0.9–15.6); p = 0.68]. Evening salivary cortisol was similar between the two groups [acyanotic 0.9 nmol/L (0.2–8.5); cyanotic 1.4 nmol/L (0.5–14.9); p = 0.32]. Both cyanotic and acyanotic groups demonstrated an intact diurnal rhythm. In conclusion, chronic hypoxia secondary to cyanotic CHD does not affect the circadian rhythm of the HPA axis. By 12 weeks of age, infants with hypoxia secondary to cyanotic CHD have a normal cortisol diurnal rhythm.


Similar content being viewed by others
References
J.I. Hoffman, S. Kaplan, The incidence of congenital heart disease. J. Am. Coll. Cardiol. 39(12), 1890–1900 (2002)
Y. Hamada, K. Kawachi, N. Tsunooka, Y. Nakamura, S. Takano, H. Imagawa, Capillary leakage in cardiac surgery with cardiopulmonary bypass. Asian Cardiovasc Thorac Ann 12(3), 193–197 (2004)
R.S. Boneva, L.D. Botto, C.A. Moore, Q. Yang, A. Correa, J.D. Erickson, Mortality associated with congenital heart defects in the United States: trends and racial disparities. Circulation 103(19), 2376–2381 (2001)
R.H. Feldt, J.C. Ewert, G.B. Stickler, W.H. Weidman, Children with congenital heart disease. Motor development, intelligence. Am. J. Dis. Child. 117(3), 281–287 (1969)
A. Silbert, P.H. Wolff, B. Mayer, A. Rosenthal, A.S. Nadas, Cyanotic heart disease, psychological development. Pediatrics 43(2), 192–200 (1969)
R.B. Aisenberg, A. Rosenthal, A.S. Nadas, P.H. Wolff, Developmental delay in infants with congenital heart disease. Correlation with hypoxemia and congestive heart failure. Pediatr. Cardiol. 3(2), 133–137 (1982)
C. Samango-Sprouse, E.C. Suddaby, Developmental concerns in children with congenital heart disease. Curr. Opin. Cardiol. 12(1), 91–98 (1997)
T. Kodama, N. Shimizu, N. Yoshikawa et al., Role of the glucocorticoid receptor for regulation of hypoxia-dependent gene expression. J. Biol. Chem. 278(35), 33384–33391 (2003)
S. Kliegman et al., Nelson Textbook of pediatrics, 18th edn. (Saunders Elsevier, Philadelphia, 2007), pp. 2352–2353
E.D. Bruder, J.K. Taylor, K.J. Kamer, H. Raff, Development of the ACTH and corticosterone response to acute hypoxia in the neonatal rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295(4), R1195–R1203 (2008)
H. Raff, J.J. Lee, E.P. Widmaier, M.K. Oaks, W.C. Engeland, Basal and adrenocorticotropin-stimulated corticosterone in the neonatal rat exposed to hypoxia from birth: modulation by chemical sympathectomy. Endocrinology 145(1), 79–86 (2004)
K. Fujimori, A. Takanashi, C. Endo, A. Sato, Stress hormone responses during 24-hour hypoxemia in preterm goat fetus. Tohoku J. Exp. Med. 215(2), 189–197 (2008)
H. Raff, L. Jacobson, W.E. Cullinan, Augmented hypothalamic corticotrophin-releasing hormone mRNA, corticosterone responses to stress in adult rats exposed to perinatal hypoxia. J. Neuroendocrinol. 19(11), 907–912 (2007)
H. Raff, L. Jacobson, W.E. Cullinan, Elevated corticosterone and inhibition of ACTH responses to CRH and ether in the neonatal rat: effect of hypoxia from birth. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285(5), R1224–R1230 (2003)
D. Zayour, S.T. Azar, N. Azar et al., Endocrine changes in a rat model of chronic hypoxia mimicking cyanotic heart disease. Endocr. Res. 29(2), 191–200 (2003)
L.B. Santiago, S.M. Jorge, A.C. Moreira, Longitudinal evaluation of the development of salivary cortisol circadian rhythm in infancy. Clin. Endocrinol. (Oxf). 44(2), 157–161 (1996)
W.S. Gozansky, J.S. Lynn, M.L. Laudenslager, W.M. Kohrt, Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic–pituitary–adrenal axis activity. Clin. Endocrinol. (Oxf). 63(3), 336–341 (2005)
H. Raff, Salivary cortisol and the diagnosis of Cushing’s syndrome: a coming of age. Endocrine 41(3), 353–354 (2012)
Z.E. Belaya, A.V. Iljin, G.A. Melnichenko et al., Diagnostic performance of late-night salivary cortisol measured by automated electrochemiluminescence immunoassay in obese and overweight patients referred to exclude Cushing’s syndrome. Endocrine 41(3), 494–500 (2012)
C. de Weerth, J. Jansen, M.H. Vos, I. Maitimu, E.G. Lentjes, A new device for collecting saliva for cortisol determination. Psychoneuroendocrinology 32(8–10), 1144–1148 (2007)
H. Raff, P.J. Homar, D.P. Skoner, New enzyme immunoassay for salivary cortisol. Clin. Chem. 49(1), 203–204 (2003)
K.J. Jenkins, K. Gauvreau, J.W. Newburger, T.L. Spray, J.H. Moller, L.I. Iezzoni, Consensus-based method for risk adjustment for surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 123(1), 110–118 (2002)
H. Raff, J.L. Raff, J.W. Findling, Late-night salivary cortisol as a screening test for Cushing’s syndrome. J. Clin. Endocrinol. Metab. 83(8), 2681–2686 (1998)
F.A. Scheer, B. Van Paassen, G.A. Van Montfrans et al., Human basal cortisol levels are increased in hospital compared to home setting. Neurosci. Lett. 333(2), 79–82 (2002)
K.R. Plumpton, B.J. Anderson, J. Beca, Thyroid hormone and cortisol concentrations after congenital heart surgery in infants younger than 3 months of age. Intensive Care Med. 36(2), 321–328 (2010)
H. Raff, J. Shinsako, L.C. Keil, M.F. Dallman, Vasopressin, ACTH, and corticosteroids during hypercapnia and graded hypoxia in dogs. Am. J. Physiol. 244(5), E453–E458 (1983)
R.W. Jones, J.H. Baumer, M.C. Joseph, E.A. Shinebourne, Arterial oxygen tension and response to oxygen breathing in differential diagnosis of congenital heart disease in infancy. Arch. Dis. Child. 51(9), 667–673 (1976)
H.J. Schmitt, W.H. Schuetz, P.A. Proeschel, C. Jaklin, Accuracy of pulse oximetry in children with cyanotic congenital heart disease. J. Cardiothorac. Vasc. Anesth. 7(1), 61–65 (1993)
F. Moncloa, E. Pretell, Cortisol secretion rate, ACTH and methopyrapone tests in high altitude native residents. J. Clin. Endocrinol. Metab. 24, 915–918 (1964)
F. Moncloa, J. Donayre, L.A. Sobrevilla, R. Guerra-García, Endocrine studies at high altitude II Adrenal cortical function in sea level natives exposed to high altitudes (4300 meters for 2 weeks. J. Clin. Endocrinol. Metab. 25(12), 1640–1642 (1965)
J.J. Larsen, J.M. Hansen, N.V. Olsen, H. Galbo, F. Dela, The effect of altitude hypoxia on glucose homeostasis in men. J. Physiol. 504(Pt 1), 241–249 (1997)
Acknowledgments
The authors thank the Herma Heart Center at the Children’s Hospital of Wisconsin; Jane Lee MD, Martin Wakeham MD, Karen Marcdante MD, Mary Dahmer, PhD, Mary Kasch, and Sara Rademacher at the Medical College of Wisconsin; and Peter Homar and the Endocrine Research Laboratory at the Aurora St. Luke’s Medical Center. The authors also thank the Elaine Kohler Fund and the Aurora Health Care Patient-Centered Research.
Conflict of interest
The authors of this study report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caprirolo, G., Ghanayem, N.S., Murkowski, K. et al. Circadian rhythm of salivary cortisol in infants with congenital heart disease. Endocrine 43, 214–218 (2013). https://doi.org/10.1007/s12020-012-9791-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9791-z