Abstract
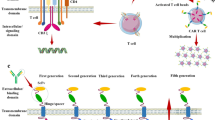
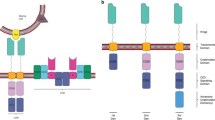
Glioblastoma (GBM) are the most common and aggressive primary brain tumors in adults. Current mainstay treatments include surgery, chemotherapy, and radiation; however, these are ineffective. As a result, immunotherapy treatment strategies are being developed to harness the body’s natural defense mechanisms against gliomas. Adoptive cell therapy with chimeric antigen receptor (CAR) T cells uses patients’ own T cells that are genetically modified to target tumor-associated antigens. These cells are harvested from patients, engineered to target specific proteins expressed by the tumor and re-injected into the patient with the goal of destroying tumor cells. In this mini review, we outline the history of CAR T cell therapy, describe current antigen targets, and review challenges this treatment faces specifically in targeting GBM.

Similar content being viewed by others
Data Availability
N/A.
Code Availability
N/A.
References
Abramson, J. S., McGree, B., Noyes, S., et al. (2017). Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. New England Journal of Medicine, 377(8), 783–784.
Ahmed, N., Brawley, V., Hegde, M., et al. (2017). HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: A phase 1 dose-escalation trial. JAMA Oncology, 3(8), 1094–1101.
Ahmed, N., Salsman, V. S., Kew, Y., et al. (2010). HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clinical Cancer Research, 16(2), 474–485.
Almasbak, H., Aarvak, T., & Vemuri, M. C. (2016). CAR T cell therapy: A game changer in cancer treatment. Journal of Immunology Research, 2016, 5474602.
Bielamowicz, K., Fousek, K., Byrd, T. T., et al. (2018). Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro-Oncology, 20(4), 506–518.
Brown, C. E., Alizadeh, D., Starr, R., et al. (2016). Regression of glioblastoma after chimeric antigen receptor T-cell therapy. New England Journal of Medicine, 375(26), 2561–2569.
Brown, C. E., Badie, B., Barish, M. E., et al. (2015). Bioactivity and safety of IL13Ralpha2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clinical Cancer Research, 21(18), 4062–4072.
Brown, C. E., Warden, C. D., Starr, R., et al. (2013). Glioma IL13Ralpha2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS ONE, 8(10), e77769.
Brown, D. V., Stylli, S. S., Kaye, A. H., & Mantamadiotis, T. (2019). Multilayered heterogeneity of glioblastoma stem cells: Biological and clinical significance. Advances in Experimental Medicine and Biology, 1139, 1–21.
Chmielewski, M., & Abken, H. (2020). TRUCKS, the fourth-generation CAR T cells: Current developments and clinical translation. Advances in Cell and Gene Therapy, 3(3), e84.
Choi, B. D., Maus, M. V., June, C. H., & Sampson, J. H. (2019). Immunotherapy for glioblastoma: Adoptive T-cell strategies. Clinical Cancer Research, 25(7), 2042–2048.
Chong, E. A., Melenhorst, J. J., Lacey, S. F., et al. (2017). PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: Refueling the CAR. Blood, 129(8), 1039–1041.
Curran, K. J., Seinstra, B. A., Nikhamin, Y., et al. (2015). Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Molecular Therapy, 23(4), 769–778.
Dubinski, D., Wolfer, J., Hasselblatt, M., et al. (2016). CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro-Oncology, 18(6), 807–818.
Ekstrand, A. J., Sugawa, N., James, C. D., & Collins, V. P. (1992). Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proceedings of the National Academy of Sciences of the United States of America, 89(10), 4309–4313.
Firor, A. E., Jares, A., & Ma, Y. (2015). From humble beginnings to success in the clinic: Chimeric antigen receptor-modified T-cells and implications for immunotherapy. Experimental Biology and Medicine (Maywood, N.J.), 240(8), 1087–1098.
Gauthier, J., & Turtle, C. J. (2018). Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Current Research in Translational Medicine, 66(2), 50–52.
Genssler, S., Burger, M. C., Zhang, C., et al. (2016). Dual targeting of glioblastoma with chimeric antigen receptor-engineered natural killer cells overcomes heterogeneity of target antigen expression and enhances antitumor activity and survival. Oncoimmunology, 5(4), e1119354.
Goff, S. L., Morgan, R. A., Yang, J. C., et al. (2019). Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. Journal of Immunotherapy, 42(4), 126–135.
Gousias, K., Markou, M., Voulgaris, S., et al. (2009). Descriptive epidemiology of cerebral gliomas in northwest Greece and study of potential predisposing factors, 2005–2007. Neuroepidemiology, 33(2), 89–95.
Gross, G., Waks, T., & Eshhar, Z. (1989). Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences of the United States of America, 86(24), 10024–10028.
Gust, J., Hay, K. A., Hanafi, L. A., et al. (2017). Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discovery, 7(12), 1404–1419.
Hao, C., Parney, I. F., Roa, W. H., Turner, J., Petruk, K. C., & Ramsay, D. A. (2002). Cytokine and cytokine receptor mRNA expression in human glioblastomas: Evidence of Th1, Th2 and Th3 cytokine dysregulation. Acta Neuropathologica, 103(2), 171–178.
Hart, D. N., & Fabre, J. W. (1981). Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. Journal of Experimental Medicine, 154(2), 347–361.
Hegde, M., Corder, A., Chow, K. K., et al. (2013). Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Molecular Therapy, 21(11), 2087–2101.
Hombach, A., Wieczarkowiecz, A., Marquardt, T., et al. (2001). Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. The Journal of Immunology, 167(11), 6123–6131.
Hong, J. J., Rosenberg, S. A., Dudley, M. E., et al. (2010). Successful treatment of melanoma brain metastases with adoptive cell therapy. Clinical Cancer Research, 16(19), 4892–4898.
Hussain, S. F., Yang, D., Suki, D., Aldape, K., Grimm, E., & Heimberger, A. B. (2006). The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology, 8(3), 261–279.
Iorgulescu, J. B., Gokhale, P. C., Speranza, M. C., et al. (2021). Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clinical Cancer Research, 27(1), 276–287.
Jensen, M. C., Popplewell, L., Cooper, L. J., et al. (2010). Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biology of Blood and Marrow Transplantation, 16(9), 1245–1256.
Joshi, B. H., Plautz, G. E., & Puri, R. K. (2000). Interleukin-13 receptor alpha chain: A novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Research, 60(5), 1168–1172.
Kershaw, M. H., Westwood, J. A., Parker, L. L., et al. (2006). A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical Cancer Research, 12(20 Pt 1), 6106–6115.
Koka, V., Potti, A., Forseen, S. E., et al. (2003). Role of Her-2/neu overexpression and clinical determinants of early mortality in glioblastoma multiforme. American Journal of Clinical Oncology, 26(4), 332–335.
Lamers, C. H., Sleijfer, S., Vulto, A. G., et al. (2006). Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. Journal of Clinical Oncology, 24(13), e20-22.
Larjavaara, S., Mantyla, R., Salminen, T., et al. (2007). Incidence of gliomas by anatomic location. Neuro-Oncology, 9(3), 319–325.
Lee, D. W., Gardner, R., Porter, D. L., et al. (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood, 124(2), 188–195.
Liu, G., Ying, H., Zeng, G., Wheeler, C. J., Black, K. L., & Yu, J. S. (2004). HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Research, 64(14), 4980–4986.
Liu, H., Jacobs, B. S., Liu, J., et al. (2000). Interleukin-13 sensitivity and receptor phenotypes of human glial cell lines: Non-neoplastic glia and low-grade astrocytoma differ from malignant glioma. Cancer Immunology, Immunotherapy, 49(6), 319–324.
Lupu, R., Colomer, R., Kannan, B., & Lippman, M. E. (1992). Characterization of a growth factor that binds exclusively to the erbB-2 receptor and induces cellular responses. Proceedings of the National Academy of Sciences of the United States of America, 89(6), 2287–2291.
Maude, S. L., Laetsch, T. W., Buechner, J., et al. (2018). Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New England Journal of Medicine, 378(5), 439–448.
Migliorini, D., Dietrich, P. Y., Stupp, R., Linette, G. P., Posey, A. D., Jr., & June, C. H. (2018). CAR T-cell therapies in glioblastoma: A first look. Clinical Cancer Research, 24(3), 535–540.
Neelapu, S. S., Locke, F. L., Bartlett, N. L., et al. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine, 377(26), 2531–2544.
Neftel, C., Laffy, J., Filbin, M. G., et al. (2019). An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell, 178(4), 835-849 e821.
O’Rourke, D. M., Nasrallah, M. P., Desai, A., et al. (2017). A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Science Translational Medicine. https://doi.org/10.1126/scitranslmed.aaa0984
Ostrom, Q. T., Bauchet, L., Davis, F. G., et al. (2014). The epidemiology of glioma in adults: A “state of the science” review. Neuro-Oncology, 16(7), 896–913.
Park, J. H., Riviere, I., Gonen, M., et al. (2018). Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. New England Journal of Medicine, 378(5), 449–459.
Pegram, H. J., Lee, J. C., Hayman, E. G., et al. (2012). Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood, 119(18), 4133–4141.
Petersen, C. T., & Krenciute, G. (2019). Next generation CAR T cells for the immunotherapy of high-grade glioma. Frontiers in Oncology, 9, 69.
Press, M. F., Cordon-Cardo, C., & Slamon, D. J. (1990). Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene, 5(7), 953–962.
Rapoport, A. P., Stadtmauer, E. A., Binder-Scholl, G. K., et al. (2015). NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nature Medicine, 21(8), 914–921.
Ricklefs, F. L., Alayo, Q., Krenzlin, H., et al. (2018). Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Science Advances, 4(3), eaar2766.
Sampson, J. H., Heimberger, A. B., Archer, G. E., et al. (2010). Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. Journal of Clinical Oncology, 28(31), 4722–4729.
Santomasso, B. D., Park, J. H., Salloum, D., et al. (2018). Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discovery, 8(8), 958–971.
Sims, J. S., Grinshpun, B., Feng, Y., et al. (2016). Diversity and divergence of the glioma-infiltrating T-cell receptor repertoire. Proceedings of the National Academy of Sciences of the United States of America, 113(25), E3529-3537.
Sugawa, N., Ekstrand, A. J., James, C. D., & Collins, V. P. (1990). Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proceedings of the National Academy of Sciences of the United States of America, 87(21), 8602–8606.
Thaci, B., Brown, C. E., Binello, E., Werbaneth, K., Sampath, P., & Sengupta, S. (2014). Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro-Oncology, 16(10), 1304–1312.
Tu, M., Wange, W., Cai, L., Zhu, P., Gao, Z., & Zheng, W. (2016). IL-13 receptor alpha2 stimulates human glioma cell growth and metastasis through the Src/PI3K/Akt/mTOR signaling pathway. Tumour Biology, 37(11), 14701–14709.
Verhaak, R. G., Hoadley, K. A., Purdom, E., et al. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell, 17(1), 98–110.
Wang, D., Starr, R., Chang, W. C., et al. (2020). Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Science Translational Medicine. https://doi.org/10.1126/scitranslmed.aaw2672
Wikstrand, C. J., McLendon, R. E., Friedman, A. H., & Bigner, D. D. (1997). Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Research, 57(18), 4130–4140.
Acknowledgements
We thank Dr. Julie Ostberg and Ms. Andrea Lynch for their assistance in manuscript preparation.
Funding
Funding for COH clinical trial by Mustang Biol, Inc.
Author information
Authors and Affiliations
Contributions
LF drafted the manuscript. BB and CB revised the manuscript critically for important intellectual content and approved the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
Lisa Feldman has no financial relationships to disclose. None. Christine Brown receives licensing (IP and royalties) and consulting payments from Mustang Bio., Inc. Behnam Badie receives licensing (IP and royalties) payments from Mustang Bio., Inc.
Ethical Approval
N/A.
Informed Consent
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feldman, L., Brown, C. & Badie, B. Chimeric Antigen Receptor (CAR) T Cell Therapy for Glioblastoma. Neuromol Med 24, 35–40 (2022). https://doi.org/10.1007/s12017-021-08689-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-021-08689-5