Abstract
Tissue-resident memory T (TRM) cells constitute a distinct subset within the memory T cell population, serving as the vanguard against invading pathogens and antigens in peripheral non-lymphoid tissues, including the respiratory tract, intestines, and skin. Notably, TRM cells adapt to the specific microenvironment of each tissue, predominantly maintaining a sessile state with distinctive phenotypic and functional attributes. Their role is to ensure continuous immunological surveillance and protection. Recent findings have highlighted the pivotal contribution of TRM cells to the modulation of adaptive immune responses in allergic disorders such as allergic rhinitis, asthma, and dermatitis. A comprehensive understanding of the involvement of TRM cells in allergic diseases bears profound implications for allergy prevention and treatment. This review comprehensively explores the phenotypic characteristics, developmental mechanisms, and functional roles of TRM cells, focusing on their intricate relationship with allergic diseases.


Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Abbreviations
- TRM:
-
Tissue-resident memory T
- APCs:
-
Antigen-presenting cells
- TCM:
-
Central memory T
- TEM:
-
Effector memory T
- pTRM:
-
Precursor tissue-resident memory T
- TEFF:
-
Effector T
- TN:
-
Naive T
- DC:
-
Dendritic cell
- ICRs:
-
Inhibitory checkpoint receptors
- ACD:
-
Allergic contact dermatitis
- TEN:
-
Toxic epidermal necrolysis
- HDM:
-
House dust mite
- CHS:
-
Contact hypersensitivity
- DETC:
-
Dendritic epidermal T cells
- IEL:
-
Intraepithelial lymphocytes
- Th2:
-
Type 2 helper T
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- S1P:
-
Sphingosine 1-phosphate
- CCR7:
-
C-C chemokine receptor type 7
- MHC:
-
Major histocompatibility complex
References
Zheng MZM, Wakim LM (2022) Tissue resident memory T cells in the respiratory tract. Mucosal Immunol 15(3):379–88. https://doi.org/10.1038/s41385-021-00461-z
Lange J, Rivera-Ballesteros O, Buggert M (2022) Human mucosal tissue-resident memory T cells in health and disease. Mucosal Immunol 15(3):389–97. https://doi.org/10.1038/s41385-021-00467-7
Hasan MH, Beura LK (2022) Cellular interactions in resident memory T cell establishment and function. Curr Opin Immunol 74:68–75. https://doi.org/10.1016/j.coi.2021.10.005
Barros L, Ferreira C, Veldhoen M (2022) The fellowship of regulatory and tissue-resident memory cells. Mucosal Immunol 15(1):64–73. https://doi.org/10.1038/s41385-021-00456-w
Tang X, Rabin RL, Yan L (2021) A three-stage design for allergen immunotherapy trials. Allergy. https://doi.org/10.1111/all.15117
Murrison LB, Brandt EB, Myers JB, Hershey GKK (2019) Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest 129(4):1504–15. https://doi.org/10.1172/jci124612
Ji H, Hu Y, Zhang T, Wang Y, Shen L, Wang S, Chen M, Wei M, Yu G (2019) Allergic comorbidity of asthma or wheezing, allergic rhinitis, and eczema: result from 333 029 allergic children in Shanghai, China. Am J Rhinol Allergy 34(2):189–195. https://doi.org/10.1177/1945892419883238
Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos JA, Smyth GK, Bevan MJ (2012) The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. https://doi.org/10.4049/jimmunol.1201305
Schenkel JM, Fraser K, Beura LK, Pauken KE, Vezys V, Masopust D (2014) Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. https://doi.org/10.1126/science.1254536
Grau-Expósito J, Sánchez-Gaona N, Massana N, Suppi M, Astorga-Gamaza A, Perea D, Rosado J, Falcó A, Kirkegaard C, Torrella A, Planas B, Navarro J, Suanzes P, Álvarez-Sierra D, Ayora A, Sansano I, Esperalba J, Andrés C, Antón A, Cajal SRy, Almirante B, Pujol-Borrell R, Falcó V, Burgos J, Buzón MJ, Genescà M (2021) Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat Commun. https://doi.org/10.1038/s41467-021-23333-3
Gálvez-Cancino F, López E, Menares E, Díaz X, Flores C, Cáceres P, Hidalgo S, Chovar O, Alcántara-Hernández M, Borgna V, Varas-Godoy M, Salazar-Onfray F, Idoyaga J, Lladser Á (2018) Vaccination-induced skin-resident memory CD8+T cells mediate strong protection against cutaneous melanoma. Oncoimmunology. https://doi.org/10.1080/2162402x.2018.1442163
Emmanuel T, Mistegård J, Bregnhøj A, Johansen C, Iversen L (2021) Tissue-resident memory T cells in skin diseases: a systematic review. Int J Mol Sci. https://doi.org/10.3390/ijms22169004
Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P (2015) Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med 212(9):1405–14
Slütter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT (2017) Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci Immunol 2(7):eaag2031. https://doi.org/10.1126/sciimmunol.aag2031
Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS (2010) Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 207(3):553–64
Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL (2011) Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 187(11):5510–5514. https://doi.org/10.4049/jimmunol.1102243
Fu J, Sykes M (2022) Emerging concepts of tissue-resident memory T cells in transplantation. Transplantation 106(6):1132–1142. https://doi.org/10.1097/tp.0000000000004000
Enamorado M, Khouili SC, Iborra S, Sancho D (2018) Genealogy, dendritic cell priming, and differentiation of tissue-resident memory CD8+ T cells. Front Immunol. https://doi.org/10.3389/fimmu.2018.01751
Stein JV, Ruef N, Wissmann S (2021) Organ-specific surveillance and long-term residency strategies adapted by tissue-resident memory CD8+ T cells. Front Immunol. https://doi.org/10.3389/fimmu.2021.626019
Mueller SN, Mackay LK (2015) Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. https://doi.org/10.1038/nri.2015.3
Leggat JA, Gibbons DL, Haque SF, Smith AL, Wells JW, Choy K, Lloyd CM, Hayday AC, Noble A (2008) Innate responsiveness of CD8 memory T-cell populations nonspecifically inhibits allergic sensitization. J Allergy Clin Immunol 122(5):1014–1021.e4. https://doi.org/10.1016/j.jaci.2008.08.011
Cheroutre H, Madakamutil L (2005) Mucosal effector memory T cells: the other side of the coin. Cell Mol Life Sci 62(23):2853–66. https://doi.org/10.1007/s00018-005-5232-y
Dijkgraaf FE, Kok L, Schumacher TNM (2021) Formation of tissue-resident CD8+ T-cell memory. Cold Spring Harb Perspect Biol 13(8): a038117. https://doi.org/10.1101/cshperspect.a038117
Walsh DA, Borges da Silva H, Beura LK, Peng C, Hamilton SE, Masopust D, Jameson SC (2019) The functional requirement for CD69 in establishment of resident memory CD8(+) T cells varies with tissue location. J Immunol 203(4):946–55. https://doi.org/10.4049/jimmunol.1900052
Turner DL, Goldklang M, Cvetkovski F, Paik D, Trischler J, Barahona J, Cao M, Dave R, Tanna N, D’Armiento JM, Farber DL (2018) Biased generation and in situ activation of lung tissue-resident memory CD4 T cells in the pathogenesis of allergic asthma. J Immunol 200(5):1561–9. https://doi.org/10.4049/jimmunol.1700257
Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, Altemeier WA, Masopust D, Pepper M (2016) Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity 44(1):155–66. https://doi.org/10.1016/j.immuni.2015.11.004
Van Dyken SJ, Nussbaum JC, Lee J, Molofsky AB, Liang HE, Pollack JL, Gate RE, Haliburton GE, Ye CJ, Marson A, Erle DJ, Locksley RM (2016) A tissue checkpoint regulates type 2 immunity. Nat Immunol 17(12):1381–7. https://doi.org/10.1038/ni.3582
Masopust D, Soerens AG (2019) Tissue-resident T cells and other resident leukocytes. Annu Rev Immunol. https://doi.org/10.1146/annurev-immunol-042617-053214
Jha P, Das H (2017) KLF2 in regulation of NF-κB-mediated immune cell function and inflammation. Int J Mol Sci. https://doi.org/10.3390/ijms18112383
Zhong F, Lee KH, He JC (2018) Role of Krüppel-like factor-2 in kidney disease. Nephrology. https://doi.org/10.1111/nep.13456
Pernaa N, Keskitalo S, Chowdhury I, Nissinen A, Glumoff V, Keski-Filppula R, Junttila J, Eklund KK, Santaniemi W, Siitonen S, Seppänen MRJ, Vähäsalo P, Varjosalo M, Åström P, Hautala T (2022) Heterozygous premature termination in zinc-finger domain of Krüppel-like factor 2 gene associates with dysregulated immunity. Front Immunol. https://doi.org/10.3389/fimmu.2022.819929
Szabo PA, Miron M, Farber DL (2019) Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol 4(34):eaas9673. https://doi.org/10.1126/sciimmunol.aas9673
Fung HY, Teryek M, Lemenze AD, Bergsbaken T (2022) CD103 fate mapping reveals that intestinal CD103- tissue-resident memory T cells are the primary responders to secondary infection. Sci Immunol 7(77):eabl9925. https://doi.org/10.1126/sciimmunol.abl9925
Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, Kupper TS (2006) A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol 126(5):1059–70. https://doi.org/10.1038/sj.jid.5700199
Cheuk S, Schlums H, Sérézal IG, Martini E, Chiang SCC, Marquardt N, Gibbs A, Detlofsson E, Introini A, Forkel M, Höög C, Tjernlund A, Michaëlsson J, Folkersen L, Mjösberg J, Blomqvist L, Ehrström M, Ståhle M, Bryceson YT, Eidsmo L (2017) CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 46(2):287–300. https://doi.org/10.1016/j.immuni.2017.01.009
Samat AAK, Geest Jvd, Vastert SJ, Loosdregt Jv, Wijk Fv (2021) Tissue-resident memory T cells in chronic inflammation-local cells with systemic effects? Cells 10(2):409. https://doi.org/10.3390/cells10020409
Schlickum S, Sennefelder H, Friedrich M, Harms GS, Lohse MJ, Kilshaw PJ, Schön MP (2008) Integrin alpha E(CD103)beta 7 influences cellular shape and motility in a ligand-dependent fashion. Blood 112(3):619–625. https://doi.org/10.1182/blood-2008-01-134833
Floc’h AL, Jalil A, Vergnon I, Chansac BLM, Lazar V, Bismuth G, Chouaı̈b S, Mami‐Chouaib F (2007) αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med 204(3):559–570. https://doi.org/10.1084/jem.20061524
Drouillard A, Neyra A, Mathieu A, Marçais A, Wencker M, Marvel J, Belot A, Walzer T (2018) Human naive and memory T cells display opposite migratory responses to sphingosine-1 phosphate. J Immunol 200(2):551–557. https://doi.org/10.4049/jimmunol.1701278
Campbell JJ, Murphy K, Kunkel EJ, Brightling CE, Soler D, Shen Z, Boisvert J, Greenberg HB, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Butcher EC, Wu L (2001) CCR7 expression and memory T cell diversity in humans. J Immunol 166(2):877–884. https://doi.org/10.4049/jimmunol.166.2.877
Parga-Vidal L, Taggenbrock R, Beumer-Chuwonpad A, Aglmous H, Kragten NAM, Behr FM, Bovens A, Lier RAWv, Stark R, Gisbergen KPJMv (2022) Hobit and Blimp-1 regulate TRM abundance after LCMV infection by suppressing tissue exit pathways of TRM precursors. Eur J Immunol 52(7):1095–1111. https://doi.org/10.1002/eji.202149665
Khan TN, Mooster JL, Kilgore AM, Osborn JF, Nolz JC (2016) Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J Exp Med 213(6):951–66. https://doi.org/10.1084/jem.20151855
Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL (2002) CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420(6915):502–7. https://doi.org/10.1038/nature01152
Zhang N, Bevan MJ (2013) Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39(4):687-96. https://doi.org/10.1016/j.immuni.2013.08.019
Mani V, Bromley SK, Äijö T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, Griffith JW, Rahimi RA, McEntee CP, Jeffrey KL, Marangoni F, Travis MA, Lacy-Hulbert A, Luster AD, Mempel TR (2019) Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 366(6462): eaav5728. https://doi.org/10.1126/science.aav5728
Hirai T, Zenke Y, Yang Y, Bartholin L, Beura LK, Masopust D, Kaplan DH (2019) Keratinocyte-mediated activation of the cytokine TGF-β maintains skin recirculating memory CD8+ T cells. Immunity 50(5):1249–1261.e5. https://doi.org/10.1016/j.immuni.2019.03.002
Worthington JJ, Kelly A, Smedley C, Bauché D, Campbell S, Marie JC, Travis MA (2015) Integrin αvβ8-mediated TGF-β activation by effector regulatory T cells is essential for suppression of T-cell-mediated inflammation. Immunity 42(5):903–15. https://doi.org/10.1016/j.immuni.2015.04.012
Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, Carbone FR (2015) T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43(6):1101–11. https://doi.org/10.1016/j.immuni.2015.11.008
Li J, Tan J, Martino MM, Lui KO (2018) Regulatory T-cells: potential regulator of tissue repair and regeneration. Front Immunol 9:585. https://doi.org/10.3389/fimmu.2018.00585
Fiege JK, Stone IA, Fay EJ, Markman MW, Wijeyesinghe S, Macchietto MG, Shen S, Masopust D, Langlois RA (2019) The impact of TCR signal strength on resident memory T cell formation during influenza virus infection. J Immunol 203(4):936–45. https://doi.org/10.4049/jimmunol.1900093
Iborra S, Martínez-López M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, Del Fresno C, Sancho D (2016) Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires crosspriming by DNGR-1(+) dendritic cells. Immunity 45(4):847–60. https://doi.org/10.1016/j.immuni.2016.08.019
Kumar BV, Wei-hua MA, Miron M, Granot T, Guyer R, Carpenter D, Senda T, Sun X, Ho SH, Lerner H, Friedman AL, Shen Y, Farber DL (2017) Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep 20(12):2921–2934. https://doi.org/10.1016/j.celrep.2017.08.078
Pizzolla A, Nguyen THO, Smith JM, Brööks AG, Kedzierska K, Heath WR, Reading PC, Wakim LM (2017) Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2(12): eaam6970. https://doi.org/10.1126/sciimmunol.aam6970
Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM (2014) CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41(4):633–645. https://doi.org/10.1016/j.immuni.2014.09.007
Peng C, Huggins MA, Wanhainen KM, Knutson TP, Lu H, Georgiev H, Mittelsteadt KL, Jarjour NN, Wang H, Hogquist KA, Campbell DJ, Borges da Silva H, Jameson SC (2022) Engagement of the costimulatory molecule ICOS in tissues promotes establishment of CD8+ tissue-resident memory T cells. Immunity 55(1):98–114.e5. https://doi.org/10.1016/j.immuni.2021.11.017
Chu KL, Batista NV, Girard M, Watts TH (2020) Monocyte-derived cells in tissue-resident memory T cell formation. J Immunol 204(3):477–485. https://doi.org/10.4049/jimmunol.1901046
Bergsbaken T, Bevan MJ, Fink PJ (2017) Local inflammatory cues regulate differentiation and persistence of CD8+ tissue-resident memory T cells. Cell Rep 19(1):114–124. https://doi.org/10.1016/j.celrep.2017.03.031
Li P, Zhang Y, Xu Y, Cao H, Li L (2022) Characteristics of CD8+ and CD4+ tissue-resident memory lymphocytes in the gastrointestinal tract. Advanced Gut Microbiome Res 2022(181):1–12. https://doi.org/10.1155/2022/9157455
Carbone FR (2015) Tissue-resident memory T cells and fixed immune surveillance in nonlymphoid organs. J Immunol 195(1):17–22. https://doi.org/10.4049/jimmunol.1500515
Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T (2013) The developmental pathway for CD103+ CD8+ tissue-resident memory T cells of skin. Nat Immunol 14(12):1294-1301. https://doi.org/10.1038/ni.2744
Behr FM, Chuwonpad A, Stark R, van Gisbergen K (2018) Armed and ready: transcriptional regulation of tissue-resident memory CD8 T cells. Front Immunol 9:1770. https://doi.org/10.3389/fimmu.2018.01770
Cheng L, Becattini S (2022) Intestinal CD8+ tissue-resident memory T cells: From generation to function. Eur J Immunol 52(10):1547–1560. https://doi.org/10.1002/eji.202149759
Behr FM, Beumer-Chuwonpad A, Kragten NAM, Wesselink TH, Stark R, van Gisbergen KPJM (2021) Circulating memory CD8+ T cells are limited in forming CD103+ tissue‐resident memory T cells at mucosal sites after reinfection. Eur J Immunol 51(1):151–166. https://doi.org/10.1002/eji.202048737
Bullock TNJ, Yagita H (2005) Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol 174(2):710–717. https://doi.org/10.4049/jimmunol.174.2.710
Kok L, Dijkgraaf FE, Urbanus J, Bresser K, Vredevoogd DW, Cardoso RF, Perié L, Beltman JB, Schumacher TN (2020) A committed tissue-resident memory T cell precursor within the circulating CD8+ effector T cell pool. J Exp Med 217(10): e20191711. https://doi.org/10.1084/jem.20191711
Mora-Buch R, Bromley SK (2021) Discipline in stages: Regulating CD8+ resident memory T cells. Front Immunol 11:624199. https://doi.org/10.3389/fimmu.2020.624199
Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, Wang W, Pipkin ME, Goldrath AW (2017) Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature. 552(7684):253–7. https://doi.org/10.1038/nature24993
Behr FM, Kragten NAM, Wesselink TH, Nota B, Lier RAWv, Amsen D, Stark R, Hombrink P, Gisbergen KPJMv (2019) Blimp-1 rather than hobit drives the formation of tissue-resident memory CD8+ T cells in the lungs. Front Immunol 10:400. https://doi.org/10.3389/fimmu.2019.00400
Schreiner D, King CG (2018) CD4+ memory T cells at home in the tissue: mechanisms for health and disease. Front Immunol 9:2394. https://doi.org/10.3389/fimmu.2018.02394
Fonseca R, Burn TN, Gandolfo LC, Devi S, Park SL, Obers A, Evrard M, Christo SN, Buquicchio FA, Lareau CA, McDonald KM, Sandford SK, Zamudio NM, Zanluqui NG, Zaid A, Speed TP, Satpathy AT, Mueller SN, Carbone FR, Mackay LK (2022) Runx3 drives a CD8+ T cell tissue residency program that is absent in CD4+ T cells. Nat Immunol 23(8):1236–45. https://doi.org/10.1038/s41590-022-01273-4
Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, Umemura M, Kyusai A, Tomura M, Nakayama T, Woodland DL, Kohlmeier JE, Miyazawa M (2016) Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69- independent maintenance. J Exp Med 213(13):3057–3073. https://doi.org/10.1038/s41385-021-00456-w
Mazzoni A, Maggi L, Montaini G, Ramazzotti M, Capone M, Vanni A, Locatello LG, Barra G, De Palma R, Gallo O, Cosmi L, Liotta F, Annunziato F (2020) Human T cells interacting with HNSCC-derived mesenchymal stromal cells acquire tissue-resident memory like properties. Eur J Immunol 50(10):1571–1579. https://doi.org/10.1002/eji.202048544
Swarnalekha N, Schreiner D, Litzler LC, Iftikhar S, Kirchmeier D, Künzli M, Son YM, Sun J, Moreira EA, King CG (2021) T resident helper cells promote humoral responses in the lung. Sci Immunol 6(55):eabb6808. https://doi.org/10.1126/sciimmunol.abb6808
Lee J, Kim D, Min B (2022) Tissue resident Foxp3+ regulatory T cells: Sentinels and saboteurs in health and disease. Front Immunol 13:865593. https://doi.org/10.3389/fimmu.2022.865593
Sun Z, Kim JH, Kim SH, Kim HR, Zhang K, Pan Y, Ko MK, Kim BM, Chu H, Lee HR, Kim HL, Kim JH, Fu X, Hyun YM, Yun KN, Kim JY, Lee DW, Song SY, Lin CP, Clark RA, Lee KH, Kupper TS, Park CO (2021) Skin-resident natural killer T cells participate in cutaneous allergic inflammation in atopic dermatitis. J Allergy Clin Immunol 147(5):1764–77. https://doi.org/10.1016/j.jaci.2020.11.049
Zakeri N, Hall A, Swadling L, Pallett LJ, Schmidt NM, Diniz MO, Kucykowicz S, Amin OE, Gander A, Pinzani M, Davidson BR, Quaglia A, Maini MK (2022) Characterisation and induction of tissue-resident gamma delta T-cells to target hepatocellular carcinoma. Nat Commun 13(1):1372. https://doi.org/10.1038/s41467-022-29012-1
van der Veeken J, Gonzalez AJ, Cho H, Arvey A, Hemmers S, Leslie CS, Rudensky AY (2016) Memory of inflammation in regulatory T cells. Cell 166(4):977–90. https://doi.org/10.1016/j.cell.2016.07.006
Son YM, Cheon IS, Wu Y, Li C, Wang Z, Gao X, Chen Y, Takahashi Y, Fu YX, Dent AL, Kaplan MH, Taylor JJ, Cui W, Sun J (2021) Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci Immunol 6(55):eabb6852. https://doi.org/10.1126/sciimmunol.abb6852
Abuzakouk M, Feighery C, O’Farrelly C (1996) Collagenase and dispase enzymes disrupt lymphocyte surface molecules. J Immunol Methods 194(2):211–6. https://doi.org/10.1016/0022-1759(96)00038-5
Perdomo C, Zedler U, Kühl AA, Lozza L, Saikali P, Sander LE, Vogelzang A, Kaufmann SH, Kupz A (2016) Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. MBio 7(6):e01686-16. https://doi.org/10.1128/mBio.01686-16
Rahimi RA, Luster AD (2018) Chemokines: critical regulators of memory T cell development, maintenance, and function. Adv Immunol 138:71–98. https://doi.org/10.1016/bs.ai.2018.02.002
Lefevre MA, Vocanson M, Nosbaum A (2021) Role of tissue-resident memory T cells in the pathophysiology of allergic contact dermatitis. Curr Opin Allergy Clin Immunol 21(4):355–60. https://doi.org/10.1097/aci.0000000000000763
Sethi GS, Gracias D, Croft M (2022) Contribution of circulatory cells to asthma exacerbations and lung tissue-resident CD4 T cell memory. Front Immunol 13:951361. https://doi.org/10.3389/fimmu.2022.951361
Nakajima H, Takatsu K (2007) Role of cytokines in allergic airway inflammation. Int Arch Allergy Immunol 142(4):265–273. https://doi.org/10.1159/000097357
Dulek DE, Newcomb DC, Goleniewska K, Cephus J, Zhou W, Reiss S, Toki S, Ye F, Zaynagetdinov R, Sherrill TP, Blackwell TS, Moore ML, Boyd KL, Kolls JK, Peebles RS (2014) Allergic airway inflammation decreases lung bacterial burden following acute Klebsiella pneumoniae infection in a neutrophil- and CCL8-dependent manner. Infect Immun 82(9):3723–3739. https://doi.org/10.1128/iai.00035-14
Rahimi RA, Nepal K, Cetinbas M, Sadreyev RI, Luster AD (2020) Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease. J Exp Med 217(9):e20190865. https://doi.org/10.1101/2020.03.25.006858
Kobayashi T, Iijima K, Matsumoto K, Lama JK, Kita H (2023) Lung-resident CD69+ST2+ TH2 cells mediate long-term type 2 memory to inhaled antigen in mice. J Allergy Clin Immunol 152(1):167-181.e6. https://doi.org/10.1016/j.jaci.2023.01.016
Ulrich BJ, Kharwadkar R, Chu M, Pajulas A, Muralidharan C, Koh B, Fu Y, Gao H, Hayes TA, Zhou HM, Goplen NP, Nelson AS, Liu Y, Linnemann AK, Turner MJ, Licona-Limón P, Flavell RA, Sun J, Kaplan MH (2022) Allergic airway recall responses require IL-9 from resident memory CD4+ T cells. Sci Immunol 7(69):eabg9296. https://doi.org/10.1126/sciimmunol.abg9296
Gauthier M, Kale SL, Oriss TB, Gorry M, Ramonell RP, Dalton K, Ray P, Fahy JV, Seibold MA, Castro M, Jarjour N, Gaston B, Bleecker ER, Meyers DA, Moore W, Hastie AT, Israel E, Levy BD, Mauger D, Erzurum S, Comhair SA, Wenzel SE, Ray A (2023) CCL5 is a potential bridge between type 1 and type 2 inflammation in asthma. J Allergy Clin Immunol 152(1):94–106.e12. https://doi.org/10.1016/j.jaci.2023.02.028
Stevens WW, Schleimer RP, Kern RC (2016) Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract 4(4):565–72. https://doi.org/10.1016/j.jaip.2016.04.012
Tan BK, Zirkle W, Chandra RK, Lin D, Conley DB, Peters AT, Grammer LC, Schleimer RP, Kern RC (2011) Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol 1(2):88–94. https://doi.org/10.1002/alr.20025
Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, Schleimer RT, Kern RC (2009) Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy 23(2):145–8. https://doi.org/10.2500/ajra.2009.23.3284
Lin DC, Chandra RK, Tan BK, Zirkle W, Conley DB, Grammer LC, Kern RC, Schleimer RP, Peters AT (2011) Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy 25(4):205–8. https://doi.org/10.2500/ajra.2011.25.3613
Pant H, Hughes A, Miljkovic D, Schembri M, Wormald P, Macardle P, Grose R, Zola H, Krumbiegel D (2013) Accumulation of effector memory CD8+ T cells in nasal polyps. Am J Rhinol Allergy 27(5):e117-26. https://doi.org/10.2500/ajra.2013.27.3958
Ickrath P, Kleinsasser N, Ding X, Ginzkey C, Beyersdorf N, Hagen R, Kerkau T, Hackenberg S (2018) Accumulation of CD69+ tissue-resident memory T cells in the nasal polyps of patients with chronic rhinosinusitis. Int J Mol Med 42(2):1116–24. https://doi.org/10.3892/ijmm.2018.3653
Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS (2006) The vast majority of CLA+ T cells are resident in normal skin. J Immunol 176(7):4431–9. https://doi.org/10.4049/jimmunol.176.7.4431
Ogg GS, Rossjohn J, Clark RA, Moody DB (2023) CD1a and bound lipids drive T-cell responses in human skin disease. Eur J Immunol 53(10):2250333. https://doi.org/10.1002/eji.202250333
Weston WL, Bruckner A (2000) Allergic contact dermatitis. Pediatr Clin North Am 47(4):897–907, vii. https://doi.org/10.1016/s0031-3955(05)70247-9
Gadsbøll A, Jee MH, Funch AB, Alhede M, Mraz V, Weber JF, Callender LA, Carroll EC, Bjarnsholt T, Woetmann A, Ødum N, Thomsen AR, Johansen JD, Henson SM, Geisler C, Bonefeld CM (2020) Pathogenic CD8+ epidermis-resident memory T cells displace dendritic epidermal T cells in allergic dermatitis. J Invest Dermatol 140(4):806–815.e5. https://doi.org/10.1016/j.jid.2019.07.722
Gamradt P, Laoubi L, Nosbaum A, Mutez V, Lenief V, Grande S, Redoulès D, Schmitt AM, Nicolas JF, Vocanson M (2019) Inhibitory checkpoint receptors control CD8+ resident memory T cells to prevent skin allergy. J Allergy Clin Immunol 143(6):2147–2157.e9. https://doi.org/10.1016/j.jaci.2018.11.048
Nielsen MM, Witherden DA, Havran WL (2017) γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol 17(12):733–45. https://doi.org/10.1038/nri.2017.101
Toyoda T, Hashimoto K, Ogawa Y, Tohyama M, Muto Y, Murashima T, Akao K, Honma K, Tanaka A (2022) Immunohistological analysis of pathogenic infiltrates in the epidermis and liver of a patient with toxic epidermal necrolysis accompanied by vanishing bile duct syndrome. J Dermatol 49(12):1343–1347. https://doi.org/10.1111/1346-8138.16576
Hashizume H (2012) Recent progress of elucidating the mechanisms of drug hypersensitivity. Asia Pac Allergy 2(3):203–209. https://doi.org/10.5415/apallergy.2012.2.3.203
Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH (2015) Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: An Update. Am J Clin Dermatol 16(6):475–493. https://doi.org/10.1007/s40257-015-0158-0
Nassif A, Bensussan A, Dorothée G, Mami-Chouaib F, Bachot N, Bagot M, Boumsell L, Roujeau JC (2002) Drug specific cytotoxic T-cells in the skin lesions of a patient with toxic epidermal necrolysis. J Invest Dermatol 118(4):728–733. https://doi.org/10.1046/j.1523-1747.2002.01622.x
Schunkert EM, Shah PN, Divito SJ (2021) Skin resident memory T cells may play critical role in delayed-type drug hypersensitivity reactions. Front Immunol 12:654190. https://doi.org/10.3389/fimmu.2021.654190
Pan Y, Kupper TS (2018) Metabolic reprogramming and longevity of tissue-resident memory T cells. Front Immunol 9:1347. https://doi.org/10.3389/fimmu.2018.01347
Mackay LK, Stock AT, Joel Z, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T (2012) Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci USA 109(18):7037–7042. https://doi.org/10.1073/pnas.1202288109
Klicznik MM, Szenes-Nagy AB, Campbell D, Gratz IK (2018) Taking the lead-how keratinocytes orchestrate skin T cell immunity. Immunol Lett 200:43–51. https://doi.org/10.1016/j.imlet.2018.06.009
Ono S, Kabashima K (2015) Novel insights into the role of immune cells in skin and inducible skin-associated lymphoid tissue (iSALT). Allergo J Int 24:170–179. https://doi.org/10.1007/s40629-015-0065-1
Akdiş M, Trautmann A, Klunker S, Daigle I, Küçüksezer UC, Deglmann W, Disch R, Blaser K (2003) T helper (Th) 2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J 17(9):1026–1035. https://doi.org/10.1096/fj.02-1070com
Pivarcsi A, Gombert M, Dieu-Nosjean MC, Lauerma A, Kubitza R, Meller S, Rieker J, Müller A, Cunha LD, Haahtela A, Sonkoly E, Fridman W-H, Alenius H, Kemény L, Ruzicka T, Zlotnik A, Homey B (2004) CC chemokine ligand 18, an atopic dermatitis-associated and dendritic cell-derived chemokine, is regulated by staphylococcal products and allergen exposure. J Immunol 173(9):5810–5817. https://doi.org/10.4049/jimmunol.173.9.5810
Furue M, Furue M (2021) OX40L–OX40 Signaling in Atopic Dermatitis. J Clin Med 10(12):2578. https://doi.org/10.3390/jcm10122578
Sletten GBG, Halvorsen R, Egaas E, Halstensen TS (2006) Memory T cell proliferation in cow’s milk allergy after CD25+ regulatory T cell removal suggests a role for casein-specific cellular immunity in IgE-Mediated but not in non-IgE-Mediated cow’s milk allergy. Int Arch Allergy Immunol 142(3):190–198. https://doi.org/10.1159/000097021
Bangert C, Rindler K, Krausgruber T, Alkon N, Thaler FM, Kurz H, Ayub T, Demirtas D, Fortelny N, Vorstandlechner V, Bauer WM, Quint T, Mildner M, Jonak C, Elbe-Bürger A, Griss J, Bock C, Brunner PM (2021) Persistence of mature dendritic cells, TH2A, and Tc2 cells characterize clinically resolved atopic dermatitis under IL-4Rα blockade. Sci Immunol 6(55):eabe2749. https://doi.org/10.1126/sciimmunol.abe2749
Acknowledgements
Figures were created by Figdraw.
Funding
This work was supported by the National Key R&D Program of China (2022YFC2504100), the National Science Foundation of China (No. 81873689), National Science Foundation of Shanghai (No. 23ZR1458000), and Shanghai General Hospital Integrated Traditional Chinese and Western Medicine (No. ZHYY-ZXYJHZX-202118).
Author information
Authors and Affiliations
Contributions
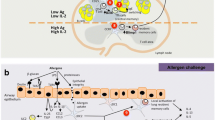
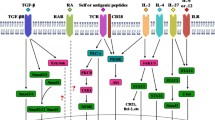
B.L., S.Z., and S.Y. co-authored the main body of the manuscript. B.L. and Y.G. were responsible for the development of Fig. 1. K.F., J.L., C.Y., J.L., X.X., and S.Y. critically reviewed the manuscript. All authors have consented to the manuscript’s submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Long, B., Zhou, S., Gao, Y. et al. Tissue-Resident Memory T Cells in Allergy. Clinic Rev Allerg Immunol 66, 64–75 (2024). https://doi.org/10.1007/s12016-024-08982-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-024-08982-8