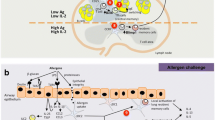
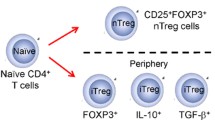
Abstract
Gamma–delta (γδ) T cells play an essential role in allergic diseases and have emerged as a potential treatment target in recent decades. To clarify the effects of γδ T cells on atopic illnesses, we reviewed the literature on the physical roles and functions of various subsets of γδ T cells, including type 1 T helper (Th1)-like, type 2 T helper- (Th2)-like, and type 17 T helper (Th17)-like γδ T cells. Mouse Vγ1 T cells increase interleukin (IL)-4 levels and trigger B cell class switching and immunoglobulin E production. Meanwhile, mouse Vγ4 T cells and human CD8lowVδ1 T cells secrete interferon-γ and exert an anti-allergy effect similar to that of Th1 cells. Moreover, mouse Vγ6 T cells produce IL-17A, while Th17-like γδ T cells enhance neutrophil and eosinophil infiltration in the acute phase of inflammation, but exert anti-inflammatory effects in the chronic phase. Human Vγ9δ2 T cells may exhibit Th1- or Th2-like characteristics in response to certain types of stimulation. In addition, the microbiota can modulate epithelial γδ T cell survival through aryl hydrocarbon receptors; these γδ T cells play crucial roles in the repair of epithelial damage, antibacterial protection, antigen tolerance, and effects of dysbiosis on allergic diseases.



Similar content being viewed by others
References
Kaiko GE, Horvat JC, Beagley KW, Hansbro PM (2008) Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology 123(3):326–338. https://doi.org/10.1111/j.1365-2567.2007.02719.x
Robinson DS (2010) The role of the T cell in asthma. J Allergy Clin Immunol 126(6):1081–1091; quiz 1092–1083. https://doi.org/10.1016/j.jaci.2010.06.025
Vatrella A, Fabozzi I, Calabrese C, Maselli R, Pelaia G (2014) Dupilumab: a novel treatment for asthma. J Asthma Allergy 7:123–130. https://doi.org/10.2147/jaa.S52387
McKenzie AN (2014) Type-2 innate lymphoid cells in asthma and allergy. Ann Am Thorac Soc 11(Suppl 5):S263–270. https://doi.org/10.1513/AnnalsATS.201403-097AW
Licona-Limón P, Kim LK, Palm NW, Flavell RA (2013) TH2, allergy and group 2 innate lymphoid cells. Nat Immunol 14(6):536–542. https://doi.org/10.1038/ni.2617
Wakashin H, Hirose K, Iwamoto I, Nakajima H (2009) Role of IL-23-Th17 cell axis in allergic airway inflammation. Int Arch Allergy Immunol 149(Suppl 1):108–112. https://doi.org/10.1159/000211382
Reyes NJ, Saban DR (2014) T helper subsets in allergic eye disease. Curr Opin Allergy Clin Immunol 14(5):477–484. https://doi.org/10.1097/aci.0000000000000088
Zheng R, Yang Q (2014) The role of the gamma delta T cell in allergic diseases. J Immunol Res 2014:963484. https://doi.org/10.1155/2014/963484
Dar AA, Patil RS, Chiplunkar SV (2014) Insights into the relationship between Toll like receptors and gamma delta T cell responses. Front Immunol 5:366. https://doi.org/10.3389/fimmu.2014.00366
Shiromizu CM, Jancic CC (2018) Gammadelta T lymphocytes: an effector cell in autoimmunity and infection. Front Immunol 9:2389. https://doi.org/10.3389/fimmu.2018.02389
Hamzaoui A, Kahan A, Ayed K, Hamzaoui K (2002) T cells expressing the gammadelta receptor are essential for Th2-mediated inflammation in patients with acute exacerbation of asthma. Mediators Inflamm 11(2):113–119. https://doi.org/10.1080/09629350220131971
Ribot JC, Lopes N, Silva-Santos B (2020) Gammadelta T cells in tissue physiology and surveillance. Nat Rev Immunol. https://doi.org/10.1038/s41577-020-00452-4
Pang DJ, Neves JF, Sumaria N, Pennington DJ (2012) Understanding the complexity of gammadelta T-cell subsets in mouse and human. Immunol 136(3):283–290. https://doi.org/10.1111/j.1365-2567.2012.03582.x
Kabelitz D, Marischen L, Oberg HH, Holtmeier W, Wesch D (2005) Epithelial defence by γδ T cells. Int Arch Allergy Immunol 137(1):73–81. https://doi.org/10.1159/000085107
Pauza CD, Liou M-L, Lahusen T, Xiao L, Lapidus RG, Cairo C, Li H (2018) Gamma delta T cell therapy for cancer: it is good to be local. Front Immunol 9(1305). https://doi.org/10.3389/fimmu.2018.01305
Wands JM, Roark CL, Aydintug MK, Jin N, Hahn YS, Cook L, Yin X, Dal Porto J, Lahn M, Hyde DM et al (2005) Distribution and leukocyte contacts of gammadelta T cells in the lung. J Leukoc Biol 78(5):1086–1096. https://doi.org/10.1189/jlb.0505244
Krug N, Erpenbeck JV, Balke K, Petschallies J, Tschernig T, Hohlfeld JM, Fabel H (2001) Cytokine profile of bronchoalveolar lavage–derived CD4, CD8, and T cells in people with asthma after segmental allergen challenge. Am J Respir Cell Mol Biol 25:125–131. https://doi.org/10.1165/ajrcmb.25.1.4194
Glanville N, Message SD, Walton RP, Pearson RM, Parker HL, Laza-Stanca V, Mallia P, Kebadze T, Contoli M, Kon OM et al (2013) GammadeltaT cells suppress inflammation and disease during rhinovirus-induced asthma exacerbations. Mucosal Immunol 6(6):1091–1100. https://doi.org/10.1038/mi.2013.3
Russano AM, Agea E, Corazzi L, Postle AD, De Libero G, Porcelli S, de Benedictis FM, Spinozzi F (2006) Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted gamma delta T cells. J Allergy Clin Immunol 117(5):1178–1184. https://doi.org/10.1016/j.jaci.2006.01.001
Yang LY, Li X, Li WT, Huang JC, Wang ZY, Huang ZZ, Chang LH, Zhang GH (2017) Vgamma1(+) gammadeltaT cells are correlated with increasing expression of eosinophil cationic protein and metalloproteinase-7 in chronic rhinosinusitis with nasal polyps inducing the formation of edema. Allergy Asthma Immunol Res 9(2):142–151. https://doi.org/10.4168/aair.2017.9.2.142
Reyes NJ, Mayhew E, Chen PW, Niederkorn JY (2011) gammadelta T cells are required for maximal expression of allergic conjunctivitis. Invest Ophthalmol Vis Sci 52(5):2211–2216. https://doi.org/10.1167/iovs.10-5959
Mengel J, Cardillo F, Aroeira LS, Williams O, Russo M, Vaz NM (1995) Anti-gamma delta T cell antibody blocks the induction and maintenance of oral tolerance to ovalbumin in mice. Immunol Lett 48(2):97–102
Bol-Schoenmakers M, Marcondes Rezende M, Bleumink R, Boon L, Man S, Hassing I, Fiechter D, Pieters RH, Smit JJ (2011) Regulation by intestinal gammadelta T cells during establishment of food allergic sensitization in mice. Allergy 66(3):331–340. https://doi.org/10.1111/j.1398-9995.2010.02479.x
Hayday AC, Vantourout P (2020) The innate biologies of adaptive antigen receptors. Annu Rev Immunol 38:487–510. https://doi.org/10.1146/annurev-immunol-102819-023144
Abeler-Dörner L, Swamy M, Williams G, Hayday AC, Bas A (2012) Butyrophilins: an emerging family of immune regulators. Trends Immunol 33(1):34–41. https://doi.org/10.1016/j.it.2011.09.007
Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP (2008) Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet 40(5):656–662. https://doi.org/10.1038/ng.108
Sutoh Y, Mohamed RH, Kasahara M (2018) Origin and evolution of dendritic epidermal T cells. Front Immunol 9:1059. https://doi.org/10.3389/fimmu.2018.01059
Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, Deban L, Cipolat S, Hart R, Iannitto ML et al (2016) Epithelia use butyrophilin-like molecules to shape organ-specific gammadelta T cell compartments. Cell 167(1):203–218 e217. https://doi.org/10.1016/j.cell.2016.08.030
Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O, Polyakova O, Roberts NA, Wesch D, Kabelitz D et al (2018) The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol 19(12):1352–1365. https://doi.org/10.1038/s41590-018-0253-5
Willcox CR, Vantourout P, Salim M, Zlatareva I, Melandri D, Zanardo L, George R, Kjaer S, Jeeves M, Mohammed F et al (2019) Butyrophilin-like 3 Directly binds a human Vγ4(+) T cell receptor using a modality distinct from clonally-restricted antigen. Immunity 51(5):813–825.e814. https://doi.org/10.1016/j.immuni.2019.09.006
Sharp LL, Jameson JM, Cauvi G, Havran WL (2005) Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol 6(1):73–79. https://doi.org/10.1038/ni1152
Strid J, Sobolev O, Zafirova B, Polic B, Hayday A (2011) The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Sci 334(6060):1293–1297. https://doi.org/10.1126/science.1211250
Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, Kaplan DH, Hayday AC, Girardi M (2008) Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol 9(2):146–154. https://doi.org/10.1038/ni1556
Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL (2009) A role for human skin-resident T cells in wound healing. J Exp Med 206(4):743–750. https://doi.org/10.1084/jem.20081787
Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H (1995) Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature (London) 373(6511):255–257
Wesch D, Glatzel A, Kabelitz D (2001) Differentiation of resting human peripheral blood gamma delta T cells toward Th1- or Th2-phenotype. Cell Immunol 212(2):110–117. https://doi.org/10.1006/cimm.2001.1850
Zhang L, Liu J, Wang E, Wang B, Zeng S, Wu J, Kimura Y, Liu B (2013) Respiratory syncytial virus protects against the subsequent development of ovalbumin-induced allergic responses by inhibiting Th2-type gammadelta T cells. J Med Virol 85(1):149–156. https://doi.org/10.1002/jmv.23435
Jin N, Miyahara N, Roark CL, French JD, Aydintug MK, Matsuda JL, Gapin L, O’Brien RL, Gelfand EW, Born WK (2007) Airway hyperresponsiveness through synergy of gammadelta} T cells and NKT cells. J Immunol 179(5):2961–2968. https://doi.org/10.4049/jimmunol.179.5.2961
Hahn YS, Ji XY, Woo SI, Choi YK, Song MS, Shin KS, Jin N, O’Brien RL, Born WK (2008) Vgamma1+ gammadelta T cells reduce IL-10-producing CD4+CD25+ T cells in the lung of ovalbumin-sensitized and challenged mice. Immunol Lett 121(2):87–92. https://doi.org/10.1016/j.imlet.2008.09.001
Schwarze J, O’Brien R, Kanehir o A, Joetham A, Gelfand EW, Köhler G, Lahn M, Takeda K, Born W, (1999) Negative regulation of airway responsiveness that is dependent on γδ T cells and independent of αβ T cells. Nat Med 5(10):1150–1156. https://doi.org/10.1038/13476
Murdoch JR, Gregory LG, Lloyd CM (2014) gammadeltaT cells regulate chronic airway inflammation and development of airway remodelling. Clin Exp Allergy 44(11):1386–1398. https://doi.org/10.1111/cea.12395
Spinozzi F, Agea E, Bistoni O, Forenza N, Monaco A, Falini B, Bassotti G, De Benedictis F, Grignani F, Bertotto A (1995) Local expansion of allergen-specific CD30+Th2-type gamma delta T cells in bronchial asthma. Mol Med 1(7):821–826
Claudia Z-A, Claude R, Solomon H, Vargaftig BB, Pablo P, Marina P (1998) Requirement for γδ T cells in allergic airway inflammation. Proc Am Assoc Adv Sci 280(5367):1265–1267
Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ (2009) Tec kinase Itk in gammadeltaT cells is pivotal for controlling IgE production in vivo. Proceedings of the National Academy of Sciences - PNAS 106(20):8308–8313. https://doi.org/10.1073/pnas.0808459106
Dong P, Zhang S, Cai M, Kang N, Hu Y, Cui L, Zhang J, He W (2014) Global characterization of differential gene expression profiles in mouse Vγ1+ and Vγ4+ γδ T cells. PLoS One 9(11):e112964. https://doi.org/10.1371/journal.pone.0112964
Horner AA, Jabara H, Ramesh N, Geha RS (1995) Gamma/delta T lymphocytes express CD40 ligand and induce isotype switching in B lymphocytes. J Exp Med 181(3):1239–1244. https://doi.org/10.1084/jem.181.3.1239
Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, O’Brien RL, Born WK (2009) The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J Immunol 183(2):849–855. https://doi.org/10.4049/jimmunol.0804104
Svensson L, Lilliehöök B, Larsson R, Bucht A (2003) Gammadelta T cells contribute to the systemic immunoglobulin E response and local B-cell reactivity in allergic eosinophilic airway inflammation. Immunology 108(1):98–108. https://doi.org/10.1046/j.1365-2567.2003.01561.x
Zhao Y, Yang J, Gao YD (2011) Altered expressions of helper T cell (Th)1, Th2, and Th17 cytokines in CD8(+) and gammadelta T cells in patients with allergic asthma. J Asthma 48(5):429–436. https://doi.org/10.3109/02770903.2011.570403
Xuekun H, Qintai Y, Yulian C, Gehua Z (2014) Correlation of gammadelta-T-cells, Th17 cells and IL-17 in peripheral blood of patients with allergic rhinitis. Asian Pac J Allergy Immunol 32(3):235–239. https://doi.org/10.12932/AP0432.32.3.2014
Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Koay HF, Nguyen HN, Mina AI, Paras T, Tavakkoli A et al (2018) gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol 19(5):464–474. https://doi.org/10.1038/s41590-018-0094-2
Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ et al (2009) CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol 10(4):427–436. https://doi.org/10.1038/ni.1717
Serre K, Silva-Santos B (2013) Molecular mechanisms of differentiation of murine pro-inflammatory γδ T cell subsets. Front Immunol 4:431. https://doi.org/10.3389/fimmu.2013.00431
McMenamin C, Pimm C, McKersey M, Holt PG (1994) Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science (American Association for the Advancement of Science) 265(5180):1869–1871. https://doi.org/10.1126/science.7916481
McMenamin C, McKersey M, Kuhnlein P, Hunig T (1950) Holt PG (1995) Gamma delta T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J Immunol 154(9):4390–4394
Bank I, Reshef A, Beniaminov M, Rosenthal E, Rechavi G, Monselise Y (1998) Role of gammadelta T cells in a patient with CD4+CD3-lymphocytosis, hypereosinophilia, and high levels of IgE. Basic and clinical immunology. https://doi.org/10.1016/s0091-6749(98)70279-9
Hacker G, Kromer S, Falk M, Heeg K, Wagner H, Pfeffer K (1992) V delta 1+ subset of human gamma delta T cells responds to ligands expressed by EBV-infected Burkitt lymphoma cells and transformed B lymphocytes. J Immunol (1950) 149(12):3984–3989
Papotto PH, Yilmaz B, Silva-Santos B (2021) Crosstalk between γδ T cells and the microbiota. Nat Microbiol 6(9):1110–1117. https://doi.org/10.1038/s41564-021-00948-2
Faustino LD, Griffith JW, Rahimi RA, Nepal K, Hamilos DL, Cho JL, Medoff BD, Moon JJ, Vignali DAA, Luster AD (2020) Interleukin-33 activates regulatory T cells to suppress innate gammadelta T cell responses in the lung. Nat Immunol 21(11):1371–1383. https://doi.org/10.1038/s41590-020-0785-3
Yang C, Kwon DI, Kim M, Im SH, Lee YJ (2021) Commensal microbiome expands Tγδ17 cells in the lung and promotes particulate matter-induced acute neutrophilia. Front Immunol 12:645741. https://doi.org/10.3389/fimmu.2021.645741
Costa MF, Bornstein VU, Candea AL, Henriques-Pons A, Henriques MG, Penido C (2012) CCL25 induces alpha(4)beta(7) integrin-dependent migration of IL-17(+) gammadelta T lymphocytes during an allergic reaction. Eur J Immunol 42(5):1250–1260. https://doi.org/10.1002/eji.201142021
Van Dyken SJ, Mohapatra A, Nussbaum JC, Molofsky AB, Thornton EE, Ziegler SF, McKenzie AN, Krummel MF, Liang HE, Locksley RM (2014) Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and gammadelta T cells. Immunity 40(3):414–424. https://doi.org/10.1016/j.immuni.2014.02.003
Murdoch JR, Lloyd CM (2010) Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing {gamma}{delta}T cells. Am J Respir Crit Care Med 182(4):464–476. https://doi.org/10.1164/rccm.200911-1775OC
Nakada EM, Shan J, Kinyanjui MW, Fixman ED (2014) Adjuvant-dependent regulation of interleukin-17 expressing γδ T cells and inhibition of Th2 responses in allergic airways disease. Respir Res 15(1):90–90. https://doi.org/10.1186/s12931-014-0090-5
Griesenauer B, Paczesny S (2017) The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol 8. https://doi.org/10.3389/fimmu.2017.00475
Thompson-Chagoyan OC, Vieites JM, Maldonado J, Edwards C, Gil A (2010) Changes in faecal microbiota of infants with cow’s milk protein allergy–a Spanish prospective case-control 6-month follow-up study. Pediatr Allergy Immunol 21(2 Pt 2):e394–400. https://doi.org/10.1111/j.1399-3038.2009.00961.x
Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, Calignano A, Khan AA, Gilbert JA, Nagler CR (2016) Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J 10(3):742–750. https://doi.org/10.1038/ismej.2015.151
Bunyavanich S, Shen N, Grishin A, Wood R, Burks W, Dawson P, Jones SM, Leung DYM, Sampson H, Sicherer S et al (2016) Early-life gut microbiome composition and milk allergy resolution. J Allergy Clin Immunol 138(4):1122–1130. https://doi.org/10.1016/j.jaci.2016.03.041
Rachid R, Stephen-Victor E, Chatila TA (2021) The microbial origins of food allergy. J Allergy Clin Immunol 147(3):808–813. https://doi.org/10.1016/j.jaci.2020.12.624
Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147(3):629–640. https://doi.org/10.1016/j.cell.2011.09.025
Zhang Z, Pu A, Yu M, Xiao W, Sun L, Cai Y, Yang H (2019) Aryl hydrocarbon receptor activation modulates γδ intestinal intraepithelial lymphocytes and protects against ischemia/reperfusion injury in the murine small intestine. Mol Med Rep 19(3):1840–1848. https://doi.org/10.3892/mmr.2019.9823
Lamas B, Natividad JM, Sokol H (2018) Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol 11(4):1024–1038. https://doi.org/10.1038/s41385-018-0019-2
Ismail AS, Behrendt CL, Hooper LV (2009) Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol 182(5):3047–3054. https://doi.org/10.4049/jimmunol.0802705
Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F et al (2011) Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A 108(21):8743–8748. https://doi.org/10.1073/pnas.1019574108
Julie J, Karen U, Nicole C, Pia Y, Elaine F, Richard B, Wendy LH (2002) A role for skin γδ T cells in wound repair. Science (American Association for the Advancement of Science) 296(5568):747–749
Boismenu R, Havran WL (1994) Modulation of epithelial cell growth by intraepithelial γδ T cells. Science (American Association for the Advancement of Science) 266(5188):1253–1255
Yang H, Antony PA, Wildhaber BE, Teitelbaum DH (2004) Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol 172(7):4151–4158. https://doi.org/10.4049/jimmunol.172.7.4151
Yaping C, Kevin C, Elaine F, Wendy LH, Richard B (2002) Protection of the intestinal mucosa by intraepithelial γδ T cells. Proceedings of the National Academy of Sciences - PNAS 99(22):14338–14343. https://doi.org/10.1073/pnas.212290499
Dupraz L, Magniez A, Rolhion N, Richard ML, Da Costa G, Touch S, Mayeur C, Planchais J, Agus A, Danne C et al (2021) Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep 36(1):109332. https://doi.org/10.1016/j.celrep.2021.109332
Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas A-K, Beck E, Wiesner J, Eberl M, Jomaa H (2001) Identification of ( E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett 509(2):317–322. https://doi.org/10.1016/S0014-5793(01)03191-X
Eberl M, Hintz M, Reichenberg A, Kollas A-K, Wiesner J, Jomaa H (2003) Microbial isoprenoid biosynthesis and human γδ T cell activation. FEBS Lett 544(1–3):4–10. https://doi.org/10.1016/s0014-5793(03)00483-6
Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser B (2009) A rapid crosstalk of human gammadelta T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog 5(2):e1000308. https://doi.org/10.1371/journal.ppat.1000308
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L et al (2010) Disordered microbial communities in asthmatic airways. PLoS One 5(1):e8578. https://doi.org/10.1371/journal.pone.0008578
Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD (2013) Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol 131(2):346–352 e341–343. https://doi.org/10.1016/j.jaci.2012.11.013
Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV et al (2007) Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 357(15):1487–1495. https://doi.org/10.1056/NEJMoa052632
He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J (2017) Gut-lung axis: The microbial contributions and clinical implications. Crit Rev Microbiol 43(1):81–95. https://doi.org/10.1080/1040841X.2016.1176988
Roduit C, Frei R, Ferstl R, Loeliger S, Westermann P, Rhyner C, Schiavi E, Barcik W, Rodriguez-Perez N, Wawrzyniak M et al (2019) High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 74(4):799–809. https://doi.org/10.1111/all.13660
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480):451–455. https://doi.org/10.1038/nature12726
Sokolowska M, Frei R, Lunjani N, Akdis CA, O’Mahony L (2018) Microbiome and asthma. Asthma Research and Practice 4(1):1. https://doi.org/10.1186/s40733-017-0037-y
Barcik W, Boutin RCT, Sokolowska M, Finlay BB (2020) The role of lung and gut microbiota in the pathology of asthma. Immunity 52(2):241–255. https://doi.org/10.1016/j.immuni.2020.01.007
Sze MA, Tsuruta M, Yang SW, Oh Y, Man SF, Hogg JC, Sin DD (2014) Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One 9(10):e111228. https://doi.org/10.1371/journal.pone.0111228
Perrone EE, Jung E, Breed E, Dominguez JA, Liang Z, Clark AT, Dunne WM, Burd EM, Coopersmith CM (2012) Mechanisms of methicillin-resistant Staphylococcus aureus pneumonia-induced intestinal epithelial apoptosis. Shock 38(1):68–75. https://doi.org/10.1097/SHK.0b013e318259abdb
Williams MR, Gallo RL (2015) The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep 15(11):65. https://doi.org/10.1007/s11882-015-0567-4
Nakatsuji T, Gallo RL (2019) The role of the skin microbiome in atopic dermatitis. Ann Allergy Asthma Immunol 122(3):263–269. https://doi.org/10.1016/j.anai.2018.12.003
Huang YJ, Marsland BJ, Bunyavanich S, O’Mahony L, Leung DY, Muraro A, Fleisher TA (2017) The microbiome in allergic disease: current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol 139(4):1099–1110. https://doi.org/10.1016/j.jaci.2017.02.007
Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, Viode C, Schmitt AM, Serre G, Simon M et al (2016) Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol 137(4):1272–1274 e1273. https://doi.org/10.1016/j.jaci.2015.07.052
Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Program NCS et al (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22(5):850–859. https://doi.org/10.1101/gr.131029.111
Myles IA, Earland NJ, Anderson ED, Moore IN, Kieh MD, Williams KW, Saleem A, Fontecilla NM, Welch PA, Darnell DA et al (2018) First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 3(9). https://doi.org/10.1172/jci.insight.120608
Paharik AE, Parlet CP, Chung N, Todd DA, Rodriguez EI, Van Dyke MJ, Cech NB, Horswill AR (2017) Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 22(6):746–756 e745. https://doi.org/10.1016/j.chom.2017.11.001
Volz T, Skabytska Y, Guenova E, Chen KM, Frick JS, Kirschning CJ, Kaesler S, Rocken M, Biedermann T (2014) Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol 134(1):96–104. https://doi.org/10.1038/jid.2013.291
Jones AL, Curran-Everett D, Leung DYM (2016) Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J Allergy Clin Immunol 137(4):1247–1248 e1243. https://doi.org/10.1016/j.jaci.2016.01.010
Merches K, Schiavi A, Weighardt H, Steinwachs S, Teichweyde N, Förster I, Hochrath K, Schumak B, Ventura N, Petzsch P et al (2020) AHR signaling dampens inflammatory signature in neonatal skin γδ T cells. Int J Mol Sci 21(6):2249
Jee MH, Mraz V, Geisler C, Bonefeld CM (2020) Gammadelta T cells and inflammatory skin diseases. Immunol Rev 298(1):61–73. https://doi.org/10.1111/imr.12913
Spidale NA, Malhotra N, Frascoli M, Sylvia K, Miu B, Freeman C, Stadinski BD, Huseby E, Kang J (2020) Neonatal-derived IL-17 producing dermal γδ T cells are required to prevent spontaneous atopic dermatitis. Elife 9. https://doi.org/10.7554/eLife.51188
De Libero G, Lau S-Y, Mori L (2015) Phosphoantigen presentation to TCR γδ cells, a conundrum getting less gray zones. Front Immunol 5:679–679. https://doi.org/10.3389/fimmu.2014.00679
Yazdanifar M, Barbarito G, Bertaina A, Airoldi I (2020) γδ T cells: the ideal tool for cancer immunotherapy. Cells 9(5). https://doi.org/10.3390/cells9051305
Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S (2020) Cancer immunotherapy with γδ T cells: many paths ahead of us. Cell Mol Immunol 17(9):925–939. https://doi.org/10.1038/s41423-020-0504-x
Acknowledgements
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/ht79V9.
Author information
Authors and Affiliations
Contributions
All authors participated in drafting and revising the article and approved the final version.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any author.
Competing Interests
The corresponding author Prof. Bor-Luen Chiang also serves as an editorial board member of Clinical Reviews in Allergy & Immunology. There was no other conflicting interest of the authors to declare relevant to this article’s content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hsu, UH., Chiang, BL. γδ T Cells and Allergic Diseases. Clinic Rev Allerg Immunol 65, 172–182 (2023). https://doi.org/10.1007/s12016-023-08966-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-023-08966-0