Abstract
Atopic dermatitis (AD) is a highly pruritic, chronic, multifactorial skin disease predisposing to bacterial and viral infections based on abnormalities of the innate and acquired immune system. The innate system quickly mobilizes an inflexible, standardized first-line response against different pathogens. Epidermal barrier dysfunction results in increased protein allergen penetration through the epidermis and predisposes to secondary skin infections. Two loss-of-function mutations in the epidermal filaggrin gene are associated with AD. Langerhans cells and inflammatory dendritic epidermal cells (IDEC) express high affinity IgE receptors, which are functional in IgE-mediated antigen presentation. Inducible antimicrobial peptides including the antiviral cathelicidin and the antibacterial beta-defensins show defective upregulation in lesional AD skin. The desmosomal protein nectin-1 is unmasked in AD lesions, thus becoming a relevant herpes simplex virus (HSV) entry receptor. Type I IFN-producing plasmacytoid dendritic cells are decreased and dysfunctional in AD skin, predisposing the patients to viral skin infections. Molluscum contagiosum virus produces a unique IL-18 binding protein to evade antiviral defense mechanisms. Innate and adaptive immunity do not simply coexist but are linked to one another in a complex network of skin immunobiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Atopic dermatitis (AD) is a clinically defined, multifactorial skin disease involving a complex genetic background, environmental trigger factors, and some characteristic skin manifestations of a distorted immune system [1, 2]. Specific clinical and therapeutic considerations formed the basis for subdivision of this disease long before our understanding of the individual pathophysiology increased within time. The human immune system is divided into the well-known acquired or adaptive immune system and the innate immune system, which is less well-understood. Although many clinical features of AD are direct consequences of the AD patient’s tendency toward Th2-skewed immune responses involving the acquired immune system, the innate immune system is also affected in AD patients [3]. Secondary cutaneous infections with viruses, bacteria, and fungi play a major role in the course of AD [3, 4]. We review the key aspects of the innate and acquired immune system and then explore current aspects of innate immunity, dendritic cell biology, and infections in AD patients.
Innate vs Adaptive Immunity
The innate immune system summarizes all inborn defense mechanisms, which protect an individual without time delay against potentially harmful substances or life forms. Inborn pathogen receptors, which are identically expressed on all immune cells, sense bacterial and viral targets with a pattern recognition technique. The advantage of an immediate response and low risk of autoimmunity is linked to the disadvantage of nonimproving responses with repeated exposure. In other words, this part of the immune system is unable to learn (Table 1). In contrast, adaptive immunity summarizes all of the acquired defense mechanisms, which form a second line of time-delayed defense against pathogens. This part of the immune system improves its reactions with repeated exposure, requires somatic recombination, and is capable of specific protein recognition instead of simple pattern recognition. The key advantage of these mechanisms involving different cell clones is that its immune responses may be built against virtually any structural protein, but the price is a considerable risk of autoimmunity.
Pathogen Recognition Receptors
The first line of innate host defense against pathogens recognizes pathogen-associated molecular patterns (PAMP) by a limited number of inborn pathogen recognition receptors (PRR), which are identical on all cells of the individual [5]. Specialized receptors of the innate immune system sense the presence of microorganisms by specific features of their bacterial or viral DNA, RNA or proteins and initiate an immune response [6]. Twelve members of the toll-like receptor (TLR) family have been identified in mammals [5]. In addition, other pathogen receptors like the mannose receptor (CD206), formyl peptide receptor (FPR)-1, and complement receptors are part of the innate immune system [7, 8]. In a wider sense, an intact epidermal barrier may also be regarded an essential component of the innate immune system. The pathogens recognized by the innate immune system involve double-stranded viral RNA (TLR-3), single-stranded viral RNA (TLR-7), CpG motifs from bacterial DNA (TLR-9), LPS from gram-negative bacterial membranes (TLR-4), FMLP from bacterial proteins (FPR-1), mannose-rich glycans from microbial glycoproteins and glycolipids (CD206), bacterial lipoteichoic acid (TLR-2), and flagellin from bacterial flagella (TLR-5) [5].
Antimicrobial Peptides
The production of antimicrobial peptides (AMP) is a powerful innate immune mechanism used by many species for fighting invading microorganisms like bacteria, fungi, and viruses after damage of the epithelial surface. A single cathelicidin and a number of defensins have been characterized in man [9]. Whereas the human beta-defensin (HBD)-1 is constitutively expressed by keratinocytes, proinflammatory stimuli induce the expression of HBD-2, HBD-3, and the cathelicidin LL-37. The frequent presence of these inducible antimicrobial peptides in psoriasis lesions corresponds nicely to our clinical experience that psoriatic skin does not show any predisposition to impetigo or other bacterial infections. A reduced production of antimicrobial peptides in the skin lesions of AD and the expression of functional variants of certain pathogen receptors have been demonstrated recently.
Dendritic Cells
Dendritic cells (DC) are a morphologically and functionally defined family of antigen presenting cells (APC), which are found in small percentages in most organs of the human body and may be further divided into a myeloid and a plasmacytoid type. Cell lineage, localization, and immunophenotype determine the nomenclature of the cell type (Fig. 1). DC are the most efficient of all APC and capable of the initiation of both primary and secondary adaptive immune responses. T cells require efficient stimulation by APC to become effector cells and participate in a pathophysiological process. The antigen uptake, processing, and presentation by DC results in an about 100-fold more effective stimulation of T cells, if antigen-specific IgE is present on the DC surface [10, 11]. Hence, DC do play a key role in the pathogenesis of AD [1, 12].
Dendritic cell nomenclature. The nomenclature of dendritic cells is determined by lineage, localization, and immunophenotype of the dendritic cell. Plasmacytoid dendritic cells are named all the same wherever these are encountered. Peripheral blood dendritic cells are divided into a myeloid and a plasmacytoid subtype. Dermal dendritic cells may belong to the CD1a expressing dermal myeloid dendritic lineage or to the plasmacytoid dendritic subtype. Epidermal dendritic cells are subdivided after their immunophenotype into classical Langerhans cells, inflammatory dendritic epidermal cells, and epidermal plasmacytoid dendritic cells
Langerhans Cells
Langerhans cells (LC) are the DC of the normal epidermis and probably the best-characterized human DC [13]. The intraepidermal network of the LC and their dendrites is regarded a first barrier of the normal skin immune system toward the environment. LC are defined as bone marrow-derived, epidermally located, dendritic APC that contain Birbeck granules and express CD1a and MHC-class II molecules [14]. Antigen exposure may induce the migration of LC to the draining lymph node, together with a phenotypic and functional maturation [15, 16]. Topical glucocorticoid therapy as well as UV light exposure may deplete LC from the epidermis.
Inflammatory Dendritic Epidermal Cells
A second epidermal dendritic cell type is exclusively present in inflammatory skin lesions. These inflammatory dendritic epidermal cells (IDEC) are defined as epidermally located, dendritic cells that do not contain Birbeck granules but express CD1a, CD11b, and class II molecules [17]. All inflammatory skin diseases associated with a lymphohistiocytic skin infiltrate are associated with the occurrence of IDEC in the epidermis. AD, psoriasis vulgaris, allergic contact eczema, mycosis fungoides, lichen planus, Dorfman–Chanarin syndrome, Netherton syndrome, Sulzberger–Garbe disease, and others contain variable percentages of IDEC within the epidermis [13, 18, 19]. As the cytokines GM-CSF and IL-4/IL-13 are abundant in AD lesions [20–23], it is assumed that IDEC derive from peripheral blood monocytic cells invading the AD lesions.
The influx of IDEC is an early event in formation of extrinsic as well as intrinsic AD lesions [24]. In contrast, the characteristic IDEC phenotype providing the basis for diagnostic immunophenotyping [19, 25], takes a few days to develop [24]. IDEC of the IgE-associated, extrinsic AD showed a significantly higher high affinity IgE receptor (FcɛRI) expression than IDEC from intrinsic AD. In addition, the diagnostic FcɛRI/FcγRII expression ratio is significantly elevated in extrinsic but not intrinsic AD [26].
Immunophenotype and Function of IDEC in AD
IDEC express more costimulatory molecules CD80 and CD86 than LC [27]. In AD lesions, the higher expression of CD86 has been demonstrated to have a functional relevance, thus supporting the role of IDEC in the presentation of antigens in AD skin [27]. The mannose receptor CD206 is also expressed on IDEC and is functional in terms of antigen uptake of mannosylated antigens by means of mannose receptor-mediated endocytosis [7]. This mechanism may play a role in Pityrosporum ovale-associated head and neck dermatitis, a clinical subtype of AD [7]. Immature dendritic cells with a higher expression of FcɛRI, thereby resembling the IDEC phenotype, can be generated in vitro under reducing cell culture conditions [28]. This model provided evidence that FcɛRI-activated IDEC may produce proinflammatory cytokines and chemokines in situ, thus actively contributing to the switch of an initial Th2-type to a Th1-type immune response in situ [29].
Treatment of AD with the topical calcineurin inhibitor tacrolimus selectively depletes IDEC from the epidermis, reduces the FcɛRI expression, and the stimulatory capacity of LC and IDEC [30]. This selective IDEC depletion phenomenon can also be induced by the topical calcineurin inhibitor pimecrolimus [31]. Topical hydrocortisone treatment increases the rate of early apoptotic DC in situ, whereas topical tacrolimus does not [32]. Both agents decrease the expression of the costimulatory molecules CD80 and CD86 on epidermal DC [32].
Plasmacytoid Dendritic Cells
Plasmacytoid dendritic cells (PDC) are a dendritic cell subset first described in the blood (CD123+++, BDCA-2+, HLA-DR+++) and make up 0.1% of peripheral blood mononuclear cells [33]. Originally termed DC-2 based on its intrinsic activity to induce Th2 responses [34] and regulatory T cells [35], PDC produce large amounts of type I interferon (IFN-α and IFN-β) upon viral infection [36]. The presence of PDC in human skin was first shown in cutaneous lupus erythematosus [37] and demonstrated shortly thereafter in psoriasis, contact dermatitis, and AD [38]. Whereas psoriasis, contact dermatitis, and especially lupus erythematosus contain relatively high numbers of PDC, only few PDC can be detected in AD lesions [38]. The selective lack of PDC in AD lesions is regarded as one of the reasons for the high susceptibility of AD patients to viral skin infections such as eczema herpeticum [3]. Crosslinking of FcɛRI on PDC reduces type I interferon production induced by TLR-9 induction [39] and the stimulatory capacity of PDC [40]. Immunostimulatory CpG oligonucleotides and imiquimod work by direct stimulation of PRR expressed on PDC.
Natural Killer Cells
Natural killer cells (NK cells) account for 10–15% of all peripheral blood lymphocytes and are important effector cells of the innate immune system, as they may lyse microbial infected target cells without a need for prior activation. Killing is mediated by release of cytoplasmic granules containing the membrane-attacking perforin and the protease granzyme. NK cells are identified by expression of CD56 but lack CD3, CD19 and T cell receptors on their cell surface [41] and may release a variety of cytokines such as IFN-γ, TNF-α, GM-CSF, IL-5 and IL-8 [42]. The number of circulating NK cells is reduced in AD [42, 43], whereas in AD lesions, NK cells have been detected in close contact to DC [44]. Coculture of Malassezia antigen-stimulated DC with NK cells may increase DC numbers in vitro [45], whereas cell contact-dependent preferential apoptosis of NK cells by activated monocytes has also been described in AD patients [43]. This may deviate immune responses toward a Th2 pattern and increase the susceptibility to infection in AD [43].
Impaired Epidermal Barrier Function in Atopic Dermatitis
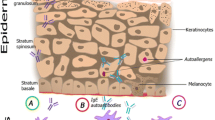
Lesional AD shows numerous histologically and immunobiologically well-characterized changes compared to normal human skin. These include a thick but dysfunctional epidermal layer, a pronounced lymphohistiocytic infiltrate, and a lack of inducible antimicrobial peptides [46, 47]. Nonlesional AD skin differs from normal human skin (Fig. 2) by high transepidermal water loss, low sebum secretion, and minimal signs of an inflammatory infiltrate [48], resulting in an increased penetration of protein allergens or other bioactive substances such as pollen-associated lipid mediators through the impaired epidermal barrier [49]. The standardized atopy patch test technique of the European Task Force on Atopic Dermatitis is a highly specific procedure and relies on intact protein allergen penetration through clinically uninvolved skin without prior destruction of the epidermal barrier by tape stripping or other procedures [50].
Histological characteristics of normal human and atopic dermatitis skin. Normal human skin (NS) shows a thin but intact and functional epidermal layer, low transepidermal water loss, and sufficient lipid baseline secretion, which results in low protein allergen penetration. Atopic dermatitis skin (AD) shows a thick but dysfunctional epidermal layer, high transepidermal water loss, and low sebum secretion, which facilitates protein allergen penetration. A scattered lymphohistiocytic infiltrate is present throughout the eczematous lesion
Recently, two loss-of-function mutations of the filaggrin gene, a filament-aggregating protein that is important in the formation of an intact stratum corneum, were found to cause ichthyosis vulgaris [51], an autosomal dominant inherited skin disease with a very dry and scaly skin including the palms and soles but sparing the flexural areas. More than one-third of all patients diagnosed with ichthyosis vulgaris suffer from AD, whereas about 2–4% of all AD patients are known to have ichthyosis vulgaris [52], implicating a connection between both diseases. It was no surprise that these two loss-of-function mutations, as well as another rare mutation in the filaggrin gene, were then found to be strong predisposing factors for AD [53–55]. Thus, an epidermal barrier defect seems to have a prominent role and is probably a primary event in AD, also predisposing to secondary infections of the skin [4]. The elucidation of the molecular basis of epidermal barrier dysfunction backs up the well-established clinical relevance of daily emollient application to nonlesional skin of AD patients [56].
Staphylococcus aureus and Atopic Dermatitis
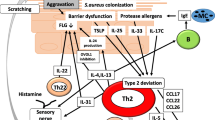
Most AD patients are colonized with Staphylococcus aureus, which may be cultured from skin lesions and, to a lesser extent, from nonlesional skin. Pathogenic factors for colonization include an impaired epidermal barrier, an altered innate immune system, a decreased bacterial clearance and an increased bacterial adhesion [4, 57]. The nasal vestibulum is the most important reservoir for recolonization of the skin [58]. Staphylococci produce a number of superantigens, as well as other exotoxins [59]. In AD, an antigen specific immune response to aero- and food allergens involving IgE-mediated, facilitated antigen presentation colocalizes with the polyclonal activation of T cells driven by superantigenic properties of staphylococcal exotoxins (Fig. 3). The same exotoxins with superantigenic properties may also be a target for IgE responses in AD patients [60].
The vicious circles of immunoactivation in atopic dermatitis lesions. This model demonstrates the two vicious circles of (1) antigen-specific and (2) superantigen-driven T cell activation in atopic dermatitis lesions. 1 Epidermal antigen presenting dendritic cells such as Langerhans cells (LC) and inflammatory dendritic epidermal cells (IDEC) present antigen to T lymphocytes (T), which will activate B cells (B) to produce antigen-specific IgE that in turn may bind to high affinity IgE receptors expressed on dendritic cells and mast cells. In the presence of antigen specific IgE on the dendritic cell surface, the antigen focusing effect of IgE-mediated antigen presentation yields about 100-fold higher T cell activation than a presentation without IgE present. 2 In addition, proinflammatory cytokines produced by T cells induce spongiosis, exsudation, and enhanced Staphylococcus aureus colonization of atopic dermatitis lesions. Staphylococcal exotoxins with superantigenic properties bypass the antigen-specificity of T cell activation, stimulating all T cells sharing the same T cell receptor family. Both mechanisms lead in synergy to profound activation of the skin immune system in the atopic dermatitis lesion
The levels of HBD-2 and the cathelicidin LL-37—as well as IL-8 and inducible NO synthetase (iNOS)—were found to be reduced in AD compared to psoriasis patients [47, 61]. The combination of HBD-2 and LL-37 has a synergistic antimicrobial activity, but the concentration of HBD-2 and LL-37 in AD is too low to effectively kill S. aureus [47]. The high concentrations of IL-4 and IL-13, which are abundant in extrinsic atopic skin, inhibit HBD-2, IL-8, and iNOS gene expression [47, 61]. The key finding of differential AMP expression in AD and psoriasis was recently confirmed for HBD-2 and other substances like elafin, calgranulin C, iNOS, psoriasin and calgranulin A, but not for LL-37 [62]. An elevated IL-10 gene expression in extrinsic and intrinsic AD is associated with a decreased HBD-2 expression [63]. Using neutralizing antibodies to IL-10, the production of HBD-2 and LL-37, TNF-α, and IFN-γ could be augmented [63]. Taken together, a deficiency in AMP may be one cause for the high rate of skin infections with S. aureus in AD.
Dermcidin is another AMP with a broad spectrum of activity, including S. aureus, and no homology to other known AMP. It is constitutively expressed in eccrine sweat glands, secreted into sweat and transported to the epidermis [64]. Several dermcidin-derived peptides are significantly reduced in AD compared to healthy subjects, and AD patients with previous bacterial or viral infections show the lowest concentration [64]. AD patients do not show the significant reduction of bacterial skin surface colonization after sweating that is observed in healthy subjects [64].
The reduction of S. aureus colonization is a well-accepted therapeutic strategy for long-term management of AD, and may be achieved by the addition of antibacterial substances to the emollients recommended for daily use [56]. In addition, oral antibiotic therapy with cephalosporins or penicillins is highly useful in severe cases of impetiginized AD.
Molluscum Contagiosum Virus and Atopic Dermatitis
Lesional and nonlesional skin of AD patients is easily infected by molluscum contagiosum virus (MCV), the sole member of the molluscipoxvirus subfamily. Its replication in the cytoplasm is mediated by distinct enzymes not present in other DNA viruses. Umbilicated small skin-colored papules are the diagnostic hallmark of molluscum infection. Patients with AD have more frequently MCV infections than nonatopic individuals and also have more widespread disease with up to several hundred lesions [65]. This disseminated eruption is called eczema molluscatum (EM). Although papules are frequently confined to the eczematous lesions, autoinoculation may produce papules in other areas. There are no systemic findings. MCV has some genes encoding for unique host response evasion proteins that help it avoid antiviral defense mechanisms [66]. MCV produces a soluble interleukin-18 binding protein (IL-18BP), which inhibits the IL-18 mediated induction of IFN-γ [67]. These findings may help explain the absence of T lymphocytes and NK cells at the base of typical MCV lesions [68].
Although EM lesions resolve spontaneously, treatment speeds healing and prevents spreading by autoinoculation and heteroinoculation. The destruction of limited numbers of lesions with a small curved forceps or a ring curette is our treatment of choice. Other destructive treatment measures include cryotherapy or carbon dioxide laser vaporization [3]. Topical application of the TLR-7 agonist imiquimod or other topical immunostimulatory drugs also shows promising results [69].
Eczema Herpeticum
Primary infection with the herpes simplex virus (HSV)-1 usually occurs during childhood and is either asymptomatic or causes herpetic gingivostomatitis. Later HSV reactivation frequently presents as recurrent perioral herpes simplex (cold sore or fever blister). Patients with compromised cell-mediated immunity, as present in AD, may develop recurrent and severe HSV infections, which may be caused by either primary or secondary HSV infection [70].
Eczema herpeticum (EH) is defined as the disseminated cutaneous infection of an eczematous skin disease with HSV, which in clinical reality is almost exclusively AD [3]. Kaposi’s varicelliform eruption (KVE) describes the disseminated cutaneous infection of any skin disease with HSV or related viruses [3]. Hence, KVE includes not only infections of AD, but also Darier disease, pemphigus foliaceus, mycosis fungoides, Sézary syndrome, ichthyosis vulgaris, Hailey–Hailey disease, and burns [3]. Disseminated, distinctly monomorphic dome-shaped vesicles (Fig. 4), frequently accompanied by fever, malaise, and lymphadenopathy are the diagnostic hallmark of EH. The head and neck are most commonly affected. Associated complications include keratoconjunctivitis and viremia leading to multiple organ involvement with meningitis and encephalitis [3].
The pathogenesis of EH involves an infection of keratinocytes, followed by an antiviral immune response, which must be mounted in a Th2-skewed environment. This underlying predisposition to Th2-type responses may actually contribute to HSV overgrowth [71]. The spongiosis and disruption of the compact keratinocyte layer present in AD unmasks nectin-1, a desmosomal protein, which is one of the relevant entry receptors for HSV [72]. A pathogenic role of AMP dysregulation for EH is well-established, as physiological concentrations of the cathelicidin LL-37 show antiviral activity against HSV in vitro and there is no upregulation of LL-37 in AD lesions in vivo [73]. Moreover, the LL-37 expression is inversely correlated to the serum IgE level of EH patients [73].
PDC produce high amounts of antiviral type I IFN-α and IFN-β upon viral infection, and play a key role in the cascade of antiviral immune responses (Fig. 5). Although the number of peripheral blood PDC is increased in AD [74], skin lesions of AD harbor very few PDC compared to other inflammatory skin diseases such as psoriasis, contact dermatitis or lupus erythematosus [38]. This lack of PDC in AD lesions can be explained by dose-dependent PDC apoptosis induction caused by IL-4, an effect that is potentiated by IL-10 [34]. Moreover, crosslinking of the high affinity IgE receptor on PDC reduces the type I interferon production induced by TLR-9 activation [39, 40]. As the defense against HSV infection may critically depend on the production of antiviral type I interferons, cutaneous PDC depletion and functional impairment may also contribute to the predisposition of AD patients to viral skin infections [38, 39].
Antiviral defense mechanisms of normal and atopic dermatitis skin. Pathogenic aspects of a viral infection and the curative immune response in normal human skin, as compared to the impaired antiviral response of eczema herpeticum arising in atopic dermatitis lesions. a In normal human skin, viral DNA/RNA activates plasmacytoid dendritic cells (PDC) by binding to pathogen recognition receptors such as toll-like receptor-7/9, which initiates an interferon-α and -β (IFN) production in PDC. The B lymphocytes transform into plasma cells (PC) and produce neutralizing antibodies. A Th1-dominated immune response develops, and Th1 cytokines induce antimicrobial peptide (AMP) production in keratinocytes (KC). In addition, natural killer cells (NK) will lyse viral infected cells. b In atopic dermatitis lesions, PDC apoptosis is rapidly induced by abundantly present Th2 cytokines from the lymphohistiocytic infiltrate. Crosslinking of IgE receptors on the remaining few PDC weakens their interferon-α and -β (IFN) production. The B lymphocytes transform into IgE-producing plasma cells (PC) and produce IgE antibodies binding to dendritic cells (PDC, IDEC) and mast cells. The Th2 cytokines lead to the generation of IgE-bearing inflammatory dendritic epidermal cells (IDEC), downregulate protective antimicrobial peptide (AMP) generation in keratinocytes, and induce apoptosis of natural killer (NK) cells
The keystone of EH treatment is systemic antiviral chemotherapy with nucleoside analogues such as acyclovir, which should be started as soon as possible [3]. The currently recommended regimen for EH is a 7-day course of intravenous acyclovir (5–10 mg/kg per dose i.v. t.i.d.), which may be prolonged according to the clinical course of the disease [3]. Oral antibiotics are frequently given to control bacterial superinfection, whereas topical antiseptic lotions may help by drying out the vesicles and preventing bacterial superinfection. Topical antiviral agents carry a risk for contact sensitization and drug reactions [75], and are usually not required for cutaneous EH lesions, but offer advantages for prophylaxis and treatment of ocular complications. EH cases with lid lesions and reduced corneal sensitivity should be treated prophylactically, whereas patients with keratitis almost always receive combined systemic and topical therapy [3]. The administration of glucocorticosteroids in acute EH is still controversial, but an increasing number of clinicians uses topical and systemic glucocorticosteroids once acyclovir has been started.
Eczema Vaccinatum
Eczema vaccinatum is a widespread eruption of vaccinia lesions in AD or other preexisting eczematous skin diseases after immunization with vaccinia virus [76]. Because eczema vaccinatum is a clinically severe and potentially life-threatening side effect, vaccination is contraindicated in all patients currently affected by AD or having a past medical history of AD [71]. Cathelicidin but not human defensins can inhibit vaccinia virus growth in vitro, suggesting that the eczema vaccinatum susceptibility of AD patients maybe caused by cathelicidin deficiency of AD patients [77]. Vaccinia virus replicates faster in AD skin explants than in normal or psoriasis skin explants [78], and vaccinia virus-induced expression of LL-37 is reduced in AD skin [78]. This nicely demonstrates that the increased vaccinia replication corresponds to a decreased expression of LL-37. The underlying cause for both observations seems to be the cytokine milieu in AD, as IL-4 and IL-13 enhance vaccinia virus replication and downregulate LL-37 [78, 79].
Vaccinia immune globulin is the treatment of choice for eczema vaccinatum [76]. The newly produced vaccinia immune globulin preparations allow an intravenous application.
Conclusion
Recent research advances provide a better understanding of some mechanisms underlying the immune deficiency in AD patients. These include the expression and function of inducible antimicrobial peptides as well as of pathogen recognition receptors on immune cells. Innate and adaptive immunity do not simply coexist but are linked to one another in a complex network of skin immunobiology.
Abbreviations
- AD:
-
atopic dermatitis
- AMP:
-
antimicrobial peptides
- APC:
-
antigen presenting cell
- DC:
-
dendritic cell
- EH:
-
eczema herpeticum
- EM:
-
eczema molluscatum
- FcɛRI:
-
high affinity IgE receptor
- FPR-1:
-
formyl peptide receptor
- HBD:
-
human beta-defensin
- HSV:
-
herpes simplex virus
- IDEC:
-
inflammatory dendritic epidermal cell
- IFN:
-
interferon
- IL18-BP:
-
interleukin-18 binding protein
- iNOS:
-
inducible NO synthetase
- KVE:
-
Kaposi’s varicelliform eruption
- LC:
-
Langerhans cell
- MCV:
-
molluscum contagiosum virus
- NK cell:
-
natural killer cell
- PAMP:
-
pathogen-associated molecular pattern
- PDC:
-
plasmacytoid dendritic cell
- PRR:
-
pathogen recognition receptor
- S. aureus :
-
Staphylococcus aureus
- TLR:
-
toll-like receptor
References
Wollenberg A, Bieber T (2000) Atopic dermatitis: from the genes to skin lesions. Allergy 55:205–213
Leung DY, Bieber T (2003) Atopic dermatitis. Lancet 361:151–160
Wollenberg A, Wetzel S, Burgdorf WHC, Haas J (2003) Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol 112:667–674
Baker BS (2006) The role of microorganisms in atopic dermatitis. Clin Exp Immunol 144:1–9
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Kaisho T, Shizuo A (2006) Toll like receptor function and signaling. J Allergy Clin Immunol 117:979–987
Wollenberg A, Mommaas M, Oppel T, Schottdorf EM, Günther S, Moderer M (2002) Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J Invest Dermatol 118:327–334
Gordon S (2002) Pattern recognition receptors: doubling up for the innate immune response. Cell 111:927–930
Braff MH, Bardan A, Nizet V, Gallo RL (2005) Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol 125:9–13
Mudde GC, Van Reijsen FC, Boland GJ, de Gast GC, Bruijnzeel PL, Bruijnzeel-Koomen CA (1990) Allergen presentation by epidermal Langerhans’ cells from patients with atopic dermatitis is mediated by IgE. Immunology 69:335–341
Maurer D, Fiebiger E, Ebner C, Reininger B, Fischer GF, Wichlas S, Jouvin M-H, Schmitt-Egenolf M, Kraft D, Kinet J-P, Stingl G (1996) Peripheral blood dendritic cells express FcεRI as a complex composed of FcεRIα and FcεRIγ- 2 Chains and can use this Receptor for IgE-mediated Allergen presentation. J Immunol 157:607–616
von Bubnoff D, Koch S, Bieber T (2003) Dendritic cells and atopic eczema/dermatitis syndrome. Curr Opin Allergy Clin Immunol 3:353–358
Wollenberg A, Bieber T (2002) Antigen presenting cells. In: Bieber T, Leung DYM editors. Atopic dermatitis. First Edition ed. New York: Marcel Dekker; 2002 pp 267–283
Wollenberg A, Schuller E (1999) Langerhans Zellen und Immunantwort. In: Plewig G, Wolff H (eds). Fortschritte der praktischen Dermatologie und Venerologie. Springer, Berlin Heidelberg New York, pp 41–48
Schuler G, Steinman RM (1985) Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med 161:526–546
Romani N, Lenz A, Glassel H, Stanzl H, Majdic O, Fritsch P, Schuler G (1989) Cultured human Langerhans cells resemble lymphoid dendritic cells in phenotype and function. J Invest Dermatol 93:600–609
Wollenberg A, Kraft S, Hanau D, Bieber T (1996) Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J Invest Dermatol 106:446–453
Wollenberg A, Geiger E, Schaller M, Wolff H (2000) Dorfman-Chanarin syndrome in a Turkish kindred: conductor diagnosis requires analysis of multiple eosinophils. Acta Derm Venereol 80:39–43
Wollenberg A, Wen S, Bieber T (1999) Phenotyping of epidermal dendritic cells - clinical applications of a flow cytometric micromethod. Cytometry 37:147–155
Pastore S, Fanales Belasio E, Albanesi C, Chinni LM, Giannetti A, Girolomoni G (1997) Granulocyte macrophage colony-stimulating factor is overproduced by keratinocytes in atopic dermatitis. Implications for sustained dendritic cell activation in the skin. J Clin Invest 99:3009–3017
Horsmanheimo L, Harvima IT, Jarvikallio A, Harvima RJ, Naukkarinen A, Horsmanheimo M (1994) Mast cells are one major source of interleukin-4 in atopic dermatitis. Br J Dermatol 131:348–353
Akdis M, Simon HU, Weigl L, Kreyden O, Blaser K, Akdis CA (1999) Skin homing (cutaneous lymphocyte-associated antigen-positive) CD8+ T cells respond to superantigen and contribute to eosinophilia and IgE production in atopic dermatitis. J Immunol 163:466–475
van der Ploeg I, Matuseviciene G, Fransson J, Wahlgren CF, Olsson T, Scheynius A (1999) Localization of interleukin-13 gene-expressing cells in tuberculin reactions and lesional skin from patients with atopic dermatitis. Scand J Immunol 49:447–453
Kerschenlohr K, Decard S, Przybilla B, Wollenberg A (2003) Atopy patch test reactions show a rapid influx of inflammatory dendritic epidermal cells (IDEC) in extrinsic and intrinsic atopic dermatitis patients. J Allergy Clin Immunol 111:869–874
Wollenberg A, Wen S, Bieber T (1995) Langerhans cell phenotyping: A new tool for differential diagnosis of inflammatory skin diseases. Lancet 346:1626–1627
Oppel T, Schuller E, Günther S, Moderer M, Haberstok J, Bieber T, Wollenberg A (2000) Phenotyping of epidermal dendritic cells allows the differentiation between extrinsic and intrinsic form of atopic dermatitis. Br J Dermatol 143:1193–1198
Schuller E, Teichmann B, Haberstok J, Moderer M, Bieber T, Wollenberg A (2001) In situ-expression of the costimulatory molecules CD80 and CD86 on Langerhans cells and inflammatory dendritic epidermal cells (IDEC) in atopic dermatitis. Arch Dermatol Res 293:448–454
Novak N, Kraft S, Haberstok J, Geiger E, Allam P, Bieber T (2002) A reducing microenvironment leads to the generation of FcepsilonRIhigh inflammatory dendritic epidermal cells (IDEC). J Invest Dermatol 119:842–849
Novak N, Valenta R, Bohle B, Laffer S, Haberstok J, Kraft S, Bieber T (2004) FcepsilonRI engagement of Langerhans cell-like dendritic cells and inflammatory dendritic epidermal cell-like dendritic cells induces chemotactic signals and different T-cell phenotypes in vitro. J Allergy Clin Immunol 113:949–957
Wollenberg A, Sharma S, von Bubnoff D, Geiger E, Haberstok J, Bieber T (2001) Topical tacrolimus (FK506) leads to profound phenotypic and functional alterations of epidermal antigen-presenting dendritic cells in atopic dermatitis. J Allergy Clin Immunol. 107:519–525
Hoetzenecker W, Ecker R, Kopp T, Stuetz A, Stingl G, Elbe-Burger A (2005) Pimecrolimus leads to an apoptosis-induced depletion of T cells but not Langerhans cells in patients with atopic dermatitis. J Allergy Clin Immunol 115:1276–1283
Schuller E, Oppel T, Bornhövd E, Wetzel S, Wollenberg A (2004) Tacrolimus ointment causes inflammatory dendritic epidermal cell depletion but no Langerhans cell apoptosis in patients with atopic dermatitis. J Allergy Clin Immunol 114:137–143
Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M (1999) Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med 5:919–923
Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ (1999) Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183–1186
Gilliet M, Liu YJ (2002) Human plasmacytoid-derived dendritic cells and the induction of T-regulatory cells. Hum Immunol 63:1149–1155
Krug A, Rothenfusser S, Selinger S, Bock C, Kerkmann M, Battiany J, Sarris A, Giese T, Speiser D, Endres S, Hartmann G (2003) CpG-A oligonucleotides induce a monocyte-derived dendritic cell-like phenotype that preferentially activates CD8 T cells. J Immunol 170:3468–3477
Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL (2001) Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol 159:237–243
Wollenberg A, Wagner M, Günther S, Towarowski A, Tuma E, Moderer M, Rothenfusser S, Wetzel S, Endres S, Hartmann G (2002) Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol 119:1096–1102
Wollenberg A, Pavicic T, Wetzel S, Hartmann G (2005) Expression of high affinity IgE receptors on skin- and blood derived plasmacytoid dendritic cells in inflammatory skin diseases. Allergy Clin Immunol Int 2:42–44
Novak N, Allam JP, Hagemann T, Jenneck C, Laffer S, Valenta R, Kochan J, Bieber T (2004) Characterization of FcepsilonRI-bearing CD123 blood dendritic cell antigen-2 plasmacytoid dendritic cells in atopic dermatitis. J Allergy Clin Immunol 114:364–370
Farag SS, Caligiuri MA (2006) Human natural killer cell development and biology. Blood Rev 20:123–137
Aktas E, Akdis M, Bilgic S, Disch R, Falk CS, Blaser K, Akdis C, Deniz G (2005) Different natural killer (NK) receptor expression and immunoglobulin E (IgE) regulation by NK1 and NK2 cells. Clin Exp Immunol 140:301–309
Katsuta M, Takigawa Y, Kimishima M, Inaoka M, Takahashi R, Shiohara T (2006) NK cells and gamma delta+ T cells are phenotypically and functionally defective due to preferential apoptosis in patients with atopic dermatitis. J Immunol 176:7736–7744
Buentke E, Heffler LC, Wilson JL, Wallin RP, Lofman C, Chambers BJ, Ljunggren HG, Scheynius A (2002) Natural killer and dendritic cell contact in lesional atopic dermatitis skin--Malassezia-influenced cell interaction. J Invest Dermatol 119:850–857
Buentke E, D’Amato M, Scheynius A (2004) Malassezia enhances natural killer cell-induced dendritic cell maturation. Scand J Immunol 59:511–516
Rajka G (1989) Essential aspects of atopic dermatitis. Springer, Berlin Heidelberg New York
Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY (2002) Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 347:1151–1160
Mihm MC, Soter NA, Dvorak HF, Austen KF (1976) The structure of normal skin and the morphology of atopic eczema. J Invest Dermatol 67:305–312
Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, Mueller MJ, Jakob T, Behrendt H (2005) Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med 201:627–636
Darsow U, Laifaoui J, Kerschenlohr K, Wollenberg A, Przybilla B, Wüthrich B, Borelli SjF, Giusti F, Seidenari S, Drzimalla K, Simon D, Disch R, Borelli S, Devillers ACA, Oranje AP, De Raeve L, Hachem JP, Dangoisse C, Blondeel A, Song M, Breuer K, Wulf A, Werfel T, Roul S, Taieb A, Bolhaar S, Bruijnzeel-Koomen C, Brönnimann M, Braathen LR, Didierlaurent A, André C, Ring J (2004) The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy 59:1318–1325
Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, Liao H, Evans AT, Goudie DR, Lewis-Jones S, Arseculeratne G, Munro CS, Sergeant A, O’Regan G, Bale SJ, Compton JG, DiGiovanna JJ, Presland RB, Fleckman P, McLean WH (2006) Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris.Nat Genet 38:337–342
Braun-Falco O, Plewig G, Wolff H, Burgdorf W (2000) Dermatology. 3rd edn. Springer, Berlin Heidelberg New York
Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O’Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, Di Giovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S, McLean WH (2006) Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 38:441–446
Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, Klopp N, Wagenpfeil S, Zhao Y, Liao H, Lee SP, Palmer CN, Jenneck C, Maintz L, Hagemann T, Behrendt H, Ring J, Nothen MM, McLean WH, Novak N (2006) Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol 118:214–219
Sandilands A, O’Regan GM, Liao H, Zhao Y, Terron-Kwiatkowski A, Watson RM, Cassidy AJ, Goudie DR, Smith FJ, McLean WH, Irvine AD (2006) Prevalent and rare mutations in the gene encoding filaggrin cause ichthyosis vulgaris and predispose individuals to atopic dermatitis. J Invest Dermatol, in press
Darsow U, Lubbe J, Taieb A, Seidenari S, Wollenberg A, Calza A, Giusti F, Ring J (2005) Position paper on diagnosis and treatment of atopic dermatitis. J Eur Acad Dermatol Venereol 19:286–295
Leung D (2005) Superantigens, steroid insensitivity and innate immunity in atopic eczema, Acta Derm Venereol Suppl 215:11–15
Abeck D, Mempel M (1998) Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol 139(Suppl 53):13–16
Breuer K, Wittmann M, Kempe K, Kapp A, Mai U, Dittrich-Breiholz O, Kracht M, Mrabet-Dahbi S, Werfel T (2005) Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy 35:1088–1095
Ide F, Matsubara T, Kaneko M, Ichiyama T, Mukouyama T, Furukawa S (2004) Staphylococcal enterotoxin-specific IgE antibodies in atopic dermatitis. Pediatr Int 46:337–341
Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, Leung DY (2003) Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 171:3262–3269
de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, Dogan A, Hiemstra PS, van de Kerkhof PC, Schalkwijk J (2005) High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol 125:1163–1173
Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, Streib J, Wong C, Gallo RL, Leung DY (2005) Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol 125:738–745
Rieg S, Steffen H, Seeber S, Humeny A, Kalbacher H, Dietz K, Garbe C, Schittek B (2005) Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol 174:8003–8010
Solomon L, Telner P (1966) Eruptive molluscum contagiosum in atopic dermatitis. CMAJ 95:978–979
Senkevich TG, Bugert JJ, Sisler JR, Koonin EV, Darai G, Moss B (1996) Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science 273:813–816
Xiang Y, Moss B (2003) Molluscum contagiosum virus interleukin-18 (IL-18) binding protein is secreted as a full-length form that binds cell surface glycosaminoglycans through the C-terminal tail and a furin-cleaved form with only the IL-18 binding domain. J Virol 77:2623–2630
Heng MC, Steuer ME, Levy A, McMahon S, Richman M, Allen SG, Blackhart B (1989) Lack of host cellular immune response in eruptive molluscum contagiosum. Am J Dermatopathol 11:248–254
Syed TA, Goswami J, Ahmadpour OA, Ahmad SA (1998) Treatment of molluscum contagiosum in males with an analog of imiquimod 1% in cream: a placebo-controlled, double-blind study. J Dermatol 25:309–313
Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B (2003) Predisposing factors and clinical features of eczema herpeticum - a retrospective analysis of 100 cases. J Am Acad Dermatol 49:198–205
Engler RJM, Kenner J, Leung DY (2002) Smallpox vaccination: risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol 110:357–365
Yoon M, Spear PG (2002) Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J Virol 76:7203–7208
Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, Pavicic T, Boguniewicz M, Leung DY (2006) Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol 117:836–841
Uchida Y, Kurasawa K, Nakajima H, Nakagawa N, Tanabe E, Sueishi M, Saito Y, Iwamoto I (2001) Increase of dendritic cells of type 2 (DC2) by altered response to IL-4 in atopic patients. J Allergy Clin Immunol 108:1005–1011
Wollenberg A, Baldauf C, Ruëff F, Przybilla B (2000) Allergische Kontaktdermatitis und Arzneiexanthem auf Aciclovir - Kreuzreaktion auf Ganciclovir. Allergo J 9:96–99
Wollenberg A, Engler R (2004) Smallpox, vaccination and adverse reactions to smallpox. Curr Opin Allergy Clin Immunol 4:271–275
Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY (2004) Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol 172:1763–1767
Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY (2006) Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity 24:341–348
Harrison JM, Ramshaw IA (2006) Cytokines, skin, and smallpox-a new link to an antimicrobial peptide. Immunity 24:245–247
Acknowledgement
The authors thank Dr. Walter H. C. Burgdorf, Tutzing, and Dr. Kerstin Katzer, Munich, for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wollenberg, A., Klein, E. Current Aspects of Innate and Adaptive Immunity in Atopic Dermatitis. Clinic Rev Allerg Immunol 33, 35–44 (2007). https://doi.org/10.1007/s12016-007-0032-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-007-0032-9