Abstract
The pathogenesis of diabetes involves complex changes in the expression profiles of mRNA and non-coding RNAs within pancreatic islet cells. Recent progress in induced pluripotent stem cell (iPSC) technology have allowed the modeling of diabetes-associated genes. Our recent study using FOXA2-deficient human iPSC models has highlighted an essential role for FOXA2 in the development of human pancreas. Here, we aimed to provide further insights on the role of microRNAs (miRNAs) by studying the miRNA-mRNA regulatory networks in iPSC-derived islets lacking the FOXA2 gene. Consistent with our previous findings, the absence of FOXA2 significantly downregulated the expression of islet hormones, INS, and GCG, alongside other key developmental genes in pancreatic islets. Concordantly, RNA-Seq analysis showed significant downregulation of genes related to pancreatic development and upregulation of genes associated with nervous system development and lipid metabolic pathways. Furthermore, the absence of FOXA2 in iPSC-derived pancreatic islets resulted in significant alterations in miRNA expression, with 61 miRNAs upregulated and 99 downregulated. The upregulated miRNAs targeted crucial genes involved in diabetes and pancreatic islet cell development. In contrary, the absence of FOXA2 in islets showed a network of downregulated miRNAs targeting genes related to nervous system development and lipid metabolism. These findings highlight the impact of FOXA2 absence on pancreatic islet development and suggesting intricate miRNA-mRNA regulatory networks affecting pancreatic islet cell development.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is caused by the dysfunction or loss of insulin-producing pancreatic β-cells [1]. Understanding the molecular mechanisms governing human pancreas development holds potential for identifying novel strategies for DM treatment. Diverse signaling pathways and transcription factors (TFs) play essential roles in orchestrating the differentiation and maturation of endocrine pancreas [2]. While animal studies have long been instrumental in biomedical research, discrepancies in physiology and metabolism between animal models and humans hinder direct translation of findings to human diabetes pathophysiology. Differences in gene expression profiles of endocrine islet cells have been documented [3, 4]. Human pluripotent stem cells (hPSCs) present a renewable resource for generating functional insulin-producing β-cells and serve as a promising platform for disease modeling and drug discovery [5,6,7]. Recent advancements in differentiating functional β-cells from hPSCs, combined with genome-editing technologies, offer avenues to investigate diabetes-associated genes and discover novel therapeutic approaches [5,6,7].
MicroRNAs (miRNAs), a group of conserved non-coding RNAs consisting of 19–25 nucleotides, exert significant influence on diverse biological processes and are implicated in various diseases. Primarily, they function by suppressing the translation or facilitating the degradation of their target mRNAs [8]. While several miRNAs have been suggested to control multiple TFs of pancreatic β-cells during their development, many of these miRNA-mRNA interactions predicted through computational methods have yet to be confirmed through experimental validation. Numerous miRNAs have been recognized for their pivotal involvement in diabetes [9, 10], the development of all endocrine cell types [11, 12], and integral contribution to the proper functioning of mature β-cells [13, 14]. Certain miRNAs have been associated with processes relevant to type 2 diabetes (T2D), including apoptosis, response to cytokines, and insulin secretion [15]. Several studies support the idea that miRNAs play critical roles in maintaining β-cell identity [11, 13,14,15,16] and that their expression is changed in diabetic conditions [15, 17]. Also, various dietary and environmental factors have been shown to influence the miRNA profile within pancreatic islets [15].
FOXA2, a crucial TF, expression is initiated at the earliest stage of pancreatic development, and its presence continues throughout subsequent developmental phases. Recent studies conducted in our laboratory have unveiled that the absence of FOXA2 during the differentiation of induced pluripotent stem cells (iPSCs) into pancreatic and hepatic cells results in pancreatic islets’ and hepatic cells’ developmental defects [18, 19]. Furthermore, our findings have brought to light changes in mRNA profiles associated with FOXA2 deficiency that are accompanied by significant alterations in the expression of long non-coding RNAs (lncRNAs) at the pancreatic progenitor (PP) and pancreatic islet stages [20]. Recently, we have also unraveled the connection between dysregulated miRNAs and differentially expressed genes (DEGs) in PPs lacking FOXA2 [21]. However, a significant gap in knowledge exists concerning the consequences of FOXA2 deficiency on the expression profiles of miRNAs and their specific target genes within pancreatic islets. Individual miRNAs can suppress hundreds of mRNA targets, and several miRNAs frequently align to influence a specific pathway, facilitating a shared developmental result [22]. Therefore, in this study, to fully understand how miRNAs and their respective targets lead to the suppression of pancreatic islet development in the absence of FOXA2, we employed genome-wide sequencing to identify DEGs and differentially expressed miRNAs (DEmiRs) dysregulated in pancreatic islets lacking FOXA2. Through the integration analysis of DEmiRs and DEGs, we conducted a network analysis to uncover candidate miRNA-regulated genes. Our results showed that the absence of FOXA2 is linked to alterations in miRNA expression, particularly those miRNAs that target pivotal pancreatic genes, during pancreatic islet development. These shifts in miRNA expression, observed in iPSC-derived islets lacking FOXA2, may signify a disruption in the process of pancreatic differentiation.
Materials and methods
Culture and Differentiation of iPSCs into Pancreatic Islets
FOXA2 knocked out iPSCs (FOXA2–/– iPSCs) as well as their wildtype (WT) control iPSCs (WT-iPSCs) previously established and fully characterized in our laboratory were used in this study [23]. All iPSCs lines were maintained in Stemflex media (ThermoFisher Scientific) on plates coated with hESC qualified Matrigel (Corning #354,277). Differentiation of iPSCs into pancreatic islets was carried out following our previously published protocol (Supplementary Table 1) [18, 24, 25].
Immunofluorescence
The cultured cells were rinsed twice with phosphate-buffered saline (PBS) and subsequently exposed to 4% paraformaldehyde (PFA) for 15–20 min at room temperature. The cells were subjected to three consecutive 10-minutes washes with tris-buffered saline containing 0.5% Tween 20 (TBST). Subsequently, the cells were permeabilized for 15 min at room temperature using PBS containing 0.5% Triton X-100 (PBST) in two rounds, followed by an overnight blocking step at 4 °C in PBST with 6% Bovine Serum Albumin (BSA). The primary antibodies were diluted in PBST with 3% BSA and the cells were subjected to an overnight incubation with these antibodies at 4oC followed by three rinses with TBST. Afterward, the cells underwent a 1-hour incubation period with PBS-diluted secondary antibodies (1:500) at room temperature. To visualize the nuclei, the stained cells were treated with Hoechst 33,258 (1:5000 in PBS) for 5 min, followed by three PBS washes prior to imaging. Images were acquired using Olympus inverted fluorescence microscope. The used primary antibodies were anti-insulin (GN-ID4, DSHB; 1:2000) and anti-glucagon (sc-514,592, Santacruz; 1:1000). The secondary antibodies were Alexa Fluor 488 donkey anti-Rat IgG (H + L) (Thermo Fisher, A21208; 1:500) and Alexa Fluor 555 goat anti-Mouse IgG (H + L) (Thermo Fisher, A-21,422; 1:500).
Western Blotting
Twenty micrograms of total protein, extracted from a single well of a 6-well plate using a mixture of RIPA lysis buffer and protease inhibitor then quantified using Pierce BCA kit (ThermoFisher Scientific), were separated via SDS-PAGE and subsequently transferred onto PVDF membranes. Following blocking of the membranes with 10% skimmed milk in TBST, the membranes underwent an overnight incubation at 4 °C with primary antibodies diluted in 5% skimmed milk in TBST, rabbit anti-FOXA2 (1:4000, 8186 S, Cell Signaling), and mouse GAPDH (1:10,000, sc-32,233, Santa Cruz). After thorough TBST washing, horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch) were applied (1:10,000) for 1 h at room temperature, followed by additional rounds of TBST washing. Finally, the membranes were developed using the SuperSignal West Pico Chemiluminescent substrate (Pierce) and imaged using the iBright™ CL 1000 Imaging System (Invitrogen).
RNA Extraction and Expression Analysis via RT-qPCR
Total RNA was extracted using the Direct-zol™ RNA Miniprep kit (Zymo Research, USA) from a single well of 6-well plate. For gene expression analysis, cDNA was synthesized from 1 µg of RNA using the SuperScript™ IV First-Strand Synthesis System following the manufacturer’s protocol (ThermoFisher Scientific, USA). RT-qPCR using the GoTaq qPCR SYBR Green Master Mix (Promega, USA) run in triplicates was done for mRNA expression analysis. To ensure accurate quantification, average Ct values were normalized to the corresponding values from WT samples for each tested gene, with GAPDH serving as the endogenous control (used primers are listed in Supplementary Table 2).
For reverse transcription of miRNA, 10 ng of total RNA was used for first strand cDNA synthesis using miRCURY LNA RT Kit (QIAGEN, Cat. No. 339,340). Quantification of the relative miRNAs’ expression level was detected by specific miRCURY LNA miRNA PCR Assays and highly sensitive miRCURY LNA SYBR® Green PCR Kit while adjusting the reverse transcriptase product dilution to 1:30 (QIAGEN, Cat. No. 339,345). The calculation of relative miRNA expression was based on the -ΔΔCT method, where SNORD48 was employed as the endogenous control for miRNA expression normalization. Used miRCURY LNA miRNA PCR Assays are listed in Supplementary Table 2.
Assessment of the Differential Gene Expression
The quality and quantity of extracted RNA were assessed using on-chip electrophoresis with the Agilent RNA 6000 Nano Kit and Agilent 2100 Bioanalyzer, following the manufacturer’s instructions. Samples with an RNA Integrity Number (RIN) > 9.0 were selected for library preparation. In accordance with the manufacturer’s instructions, the NEBNext Poly(A) mRNA Magnetic Isolation Kit (NEB, E7490) was employed to capture mRNA, utilizing 1 µg of total RNA. The RNA-sequencing (RNA-seq) libraries were generated using the NEBNext Ultra Directional RNA Library Prep Kit (NEB, E7420L), The yield of cDNA libraries was measured using the Qubit dsDNA HS Assay Kit, and the size distribution of the cDNA libraries was determined using the Agilent 2100 Bioanalyzer DNA1000 chip. Subsequently, libraries were pooled and subjected to sequencing on the Illumina Hiseq 4000 platform, resulting in an average read count of > 8,400,000 per sample. To process the raw data, the Illumina BCL2Fastq Conversion Software v2.20 was utilized, with concurrent quality control procedures. Subsequently, using the built-in module and default settings within CLC Genomics Workbench v21.0.5, pair-end FASTQ files were aligned to the GRCh38 reference genome. For differential expression analysis, the normalized expression data represented as TPM (transcripts per million) mapped reads were imported into AltAnalyze v.2.1.3 as described before [26]. Differentially expressed genes (DEGs) were identified based on criteria where the log2 fold change (FC) exceeded 1 or was less than − 1, accompanied by a P-value < 0.05. Functional enrichment analyses encompassing Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were conducted using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) [27].
Differential miRNA Expression and Potential Target Analysis in iPSC-Derived Pancreatic Islets
miRNA library was prepared from approximately 100ng of the extracted total RNA, following the manufacturer’s guidelines provided with the library kit (E7560S, New England BioLabs Inc., USA). Monarch PCR purification kit (Biolabs, New England) used to purify the resulting amplified cDNA constructs. For miRNA analysis, FASTQ files were aligned to the miRBase v22 database (mature miRNAs), and miRNA expression (total counts) was estimated using the built-in small RNA analysis workflow with default settings in CLC Genomics Workbench 20.2, as previously described [28]. Over 70% of the annotated reads mapped to the miRBase v22 database, with more than 75% of those reads exhibiting a perfect match. Approximately 57% of miRBase-annotated miRNAs were detected in our samples. The CLC analysis pipeline did not provide information on other small RNAs, as this study focused specifically on miRNAs. Our analysis exclusively focused on reporting mature miRNAs (5p and 3p) according to miRBase v22 database annotations. To ensure accuracy, miRNA count reads were normalized using the TMM (trimmed mean of M values) method, and the resulting log2 CPM (Counts per Million) values were then subjected to subsequent differential analysis. Differentially expressed miRNAs in FOXA2–/– iPSCs compared to WT-iPSCs derived pancreatic islets were identified based on a log2 FC > 1 with a P-value < 0.05 threshold. Pathway analysis and the miRNA target filter identification were employed to unveil potential miRNA–mRNA networks, facilitated by Ingenuity Pathway Analysis (IPA) software (QIAGEN, Germany).
Statistical Analysis
Most experiments incorporated at least four biological replicates; in cases where this was not feasible, technical replicates were employed for subsequent statistical analysis. Prism 8 software was used to perform the statistical analysis, utilizing an unpaired two-tailed student’s t-test. The results are expressed as the mean ± standard deviation (SD).
Results
Profiling Alterations in Gene Expression Patterns in iPSC-Derived Pancreatic Islets Lacking FOXA2
To explore the impact of FOXA2 depletion on mRNA and miRNA expression profiles within pancreatic islets, we utilized two FOXA2–/– iPSC lines generated through CRISPR/Cas9 technology, along with their WT-iPSC control counterparts, as previously documented [18]. Using a stepwise protocol, the cell lines were differentiated into mature pancreatic islets (Fig. 1A and Supplementary Table 9). Our results demonstrated the complete absence of FOXA2 at protein level using western blotting (Fig. 1B) as well as clear demolishment in the number of INS- and GCG-expressing cells in FOXA2–/– islets (Fig. 1C). To identify the DEGs in pancreatic islets derived from both WT and FOXA2–/– iPSCs, we conducted RNA-Seq analysis. Our transcriptome analysis unveiled 719 DEGs that were significantly upregulated (Log2 FC > 1, p < 0.05), alongside 999 DEGs that were significantly downregulated (Log2 FC < − 1, p < 0.05), when comparing FOXA2–/– islets to their WT-islet counterparts (Fig. 1D, E and Supplementary Tables 3 and 4). To visually represent the distinct transcriptomic profiles between WT-islets and FOXA2–/– islets, we performed PCA, which effectively clustered the data into two separate groups, underscoring the pronounced differences (Supplementary Fig. 1A-B). Further exploration of the downregulated DEGs through GO and KEGG pathways enrichment analyses revealed strong associations with pancreatic islet genes that are critical in processes such as diabetes mellitus, insulin secretion and processing, and glucose homeostasis (Fig. 1F, left panel and Supplementary Fig. 1C). The most important downregulated DEGs that play crucial roles in pancreatic islets’ development and functionality were enlisted in Fig. 2A. Conversely, when examining the upregulated DEGs, we observed enriched pathways primarily linked to nervous system development, lipid metabolism, bile acid and cholesterol metabolism, WNT signaling pathway, and various other metabolic signaling pathways (Fig. 1F, right panel). Absence of FOXA2 was correlated with the upregulation of many genes, primarily associated with processes related to fat and cholesterol metabolism, as well as lipid and neuronal development, among other metabolic pathways (Figs. 1F and 2A, and Fig. 3A). To validate the RNA-Seq findings, we conducted RT-qPCR analysis on a selection of crucial key pancreatic DEGs (Fig. 2B). Our RT-qPCR results provided robust confirmation of the RNA-Seq data, reaffirming the significant reduction of mRNA expression in pancreatic islet genes, including INS, GCG, PPY, IAPP, ARX, NKX6.1, PDX1, GCGR, UCN3, NEUROG3, NEUROD1, NKX2.2, INSM1, PAX4, PAX6, RFX6, HES1, HES6, and PTF1A, within FOXA2–/– islets (Fig. 2B). In addition, we validated some of the significantly upregulated DEGs at mRNA levels including APOA2, APOC3, ABCC6, AFP, and OLIG3. Also, some genes known to be expressed in pancreatic islets such as LIN28A, NPY, and delta cell-specific markers, HHEX and SST were significantly upregulated (Fig. 2C and Supplementary Table 3).
Effect of FOXA2 loss on pancreatic islet differentiation. (A) Schematic representation of stepwise iPSC differentiation protocol into pancreatic islets. (B) Western blot analysis confirming the absence of FOXA2 expression in pancreatic islets derived from FOXA2–/– iPSCs (full-length blots/gels are presented in Supplementary Fig. 1). (C) Immunofluorescence showing significant reduction in the expression of INS and GCG in FOXA2–/– islets. (D) General heatmap of differentially expressed genes (DEGs) in pancreatic islets derived from WT and FOXA2–/– iPSCs. (E) Volcano plots representing the downregulated DEGs as blue dots while the red dots indicate upregulated DEGS in FOXA2–/– islets. (F) Gene ontology (GO) enrichment analysis terms for downregulated (blue) and upregulated (red) DEGs
FOXA2 loss changes transcriptomic profile in pancreatic islets lacking FOXA2. (A) Heatmap for selected pancreatic islet-associated downregulated DEGs in FOXA2–/– islets (n = 2). RNA-Seq results were validated using RT-qPCR for selected downregulated DEGs (n = 4) (B) and upregulated DEGs (n = 4) (C). Data are represented as mean ± SD
Differential miRNA expression profiling of FOXA2–/– islets and WT-islets. (A) General heatmap of dysregulated DEmiRs in FOXA2–/– islets compared to WT-islets (n = 2). (B) Volcano plot of downregulated DEmiRs as blue dots and upregulated DEmiRs as red dots. (C) Heatmap of the selected upregulated DEmiRs targeting key pancreatic islet genes (n = 2). (D) RT-qPCR validation of selected downregulated and upregulated DEmiRs (n = 4). Data are represented as mean ± SD
Altered miRNA Expression Profile in iPSC-Derived Pancreatic Islets Lacking FOXA2
To unveil the alterations in the miRNA expression profile, we conducted miRNA-Seq analysis using the same RNA samples collected from iPSC-derived pancreatic islets of FOXA2–/– and WT. Our miRNA-Seq investigation identified 61 significantly upregulated miRNAs (Log2 FC > 1, p < 0.05) and 99 significantly downregulated miRNAs (Log2 FC < − 1, p < 0.05), collectively referred to as DEmiRs, in FOXA2–/– pancreatic islets compared to WT counterparts (Fig. 3A, B). The top upregulated and downregulated DEmiRs are detailed in Supplementary Tables 5 and 6, respectively. Given the substantial suppression of β-cell development observed in the absence of FOXA2 [18], our study concentrated on the upregulated DEmiRs. Many of these DEmiRs are implicated in regulating the expression of key genes crucial for β-cell development [15, 29,30,31], and glucose regulation [15, 32] (Fig. 3C).
To validate our findings, we performed RT-qPCR analysis on selected DEmiRs. Our results demonstrated significant upregulation of miR-199a-5p, miR-199a-3p, miR-204-5p, miR-122-5p, miR-1-3p, miR-383-5p, miR-218-5p, miR-373-3p, miR-371a-5p, miR-137-3p, miR-371a-3p, miR-155-5p, miR-10b-5p, and miR-105-5p, along with significant downregulation of miR-98-5p, miR-203b-3p, let-7f-2-3p, miR-136-3p, miR-934, miR-493-3p, miR-409-3p, miR-99a-3p, miR-203a-3p, miR-340-5p, miR-542-3p, and miR-337-3p (Fig. 3D).
Upregulated DEmiRs Potentially Target Downregulated Pancreatic Genes in FOXA2 –/– Islets
To gain insights into the roles of miRNAs during pancreatic islet cell development in the lack of FOXA2, we used the miRNA target filter in Ingenuity Pathway Analysis (IPA) Software to identify the potential target genes while focusing on the upregulated DEmiRs crosslinked with the downregulated DEGs from our datasets. The target prediction analysis revealed 55 significantly upregulated DEmiRs (Log2 FC > 1, p < 0.05) targeting 756 significantly downregulated DEGs (Log2 FC < − 1, p < 0.05) in FOXA2–/– islets. Among the 756 identified targeted DEGs, 32 were selectively identified as important pancreatic islet associated DEGs, which were targeted by 45 upregulated DEmiRs (Fig. 4; Table 1). Hereafter, we focused on these selected DEGs, which are known for their pivotal roles in pancreatic islet development and function. Our investigation, with a particular emphasis on genes and TFs related to pancreatic endocrine specification, unveiled an intricate network of miRNA-mediated regulatory interactions. Notably, NEUROG3 emerged as a bona fide target for the hsa-miR-372-3p, hsa-miR-106a-3p, hsa-miR-30b-3p, and hsa-miR-887-5p, while NEUROD1 was a predicted target by hsa-miR-133a-5p, hsa-miR-1-3p, hsa-miR-372-3p, hsa-miR-2682-5p, hsa-miR-371a-5p, hsa-miR-137-3p, hsa-miR-1269b, and hsa-miR-1263. Furthermore, the NOTCH negative regulator TF, HES6, was predicted to be targeted by hsa-miR-214-3p, hsa-miR-371a-3p, hsa-miR-3085-3p, hsa-miR-6815-5p, and hsa-miR-30b-3p. Similarly, LMX1B was targeted by hsa-miR-214-3p, hsa-miR-3085-3p, hsa-miR-6815-5p, and hsa-miR-760. NKX6 TFs, including NKX6.3 and NKX6.1, were targeted by several miRNAs, including hsa-miR-214-5p, hsa-miR-133a-3p, hsa-miR-365a-5p, and hsa-miR-504-5p for NKX6.3, and hsa-miR-548w, hsa-miR-193b-5p, and hsa-miR-34b-3p for NKX6.1. Moreover, PDX1 and PAX4 were anticipated targets for hsa-miR-193b-3p. MAFF was identified as a predicted target for 8 different DEmiRs including hsa-miR-214-3p, hsa-miR-133a-3p, hsa-miR-1269b, hsa-miR-3085-3p, hsa-miR-708-5p, hsa-miR-30b-3p, hsa-miR-760, and hsa-miR-193b-5p. INSM1 was a predicted target for hsa-miR-125b-1-3p, and hsa-miR-100-5p. Additionally, ARX is predicted to be targeted by hsa-miR-204-5p, hsa-miR-372-3p, and hsa-miR-371a-3p.
Target prediction analysis of upregulated DEmiRs targeting key downregulated pancreatic islet DEGs. The target prediction network between upregulated DEmiRs and downregulated DEGs was conducted using the Ingenuity Pathway Analysis (IPA) tool. Green lines indicate experimentally observed targets, orange lines represent a high prediction confidence level, while grey lines indicate a moderate prediction confidence level
In addition to the TFs, other genes with diverse cellular roles, implicated in pancreatic islet and β-cell function, were also targeted by the significantly upregulated DEmiRs in the absence of FOXA2. For example, IGFBP1 was predicted to be targeted by hsa-miR-199a-5p, hsa-miR-2682-5p, hsa-miR-708-5p, and hsa-miR-6815-5p, while IAPP was predicted to be targeted by has-miR-143-3p, has-miR-155-5p, and has-miR-34b-3p. TRPM4 was targeted by hsa-miR-199a-5p, hsa-miR-152-5p, and hsa-miR-708-5p. SLC30A8, an important zinc transporter into insulin-containing granules [33, 34], was also targeted by a multitude of upregulated DEmiRs, including hsa-miR-214-5p, hsa-miR-383-5p, hsa-miR-145-3p, hsa-miR-143-3p, hsa-miR-1269b, hsa-miR-6815-5p, hsa-miR-30b-3p, and hsa-miR-887-5p. PCLO, which is known to be associated with diabetes [35], was predicted to be targeted by has-miR-218-5p and has-miR-3117-3p. The exocytotic gene [36, 37], STX1A, was also targeted by hsa-miR-214-5p, hsa-miR-490-3p, hsa-miR-371a-5p, hsa-miR-6815-5p, hsa-miR-30b-3p, hsa-miR-1251-3p, and hsa-miR-760. Furthermore, members of the adenylate cyclase family, including ADCY1, 2 and 7, which catalyze the cAMP generation, were targeted by hsa-miR-365a-3p, hsa-miR-137-3p, hsa-miR-708-5p, and hsa-miR-490-3p, respectively. Delta like canonical Notch ligands 1 and 4 (DLL1 and DLL4) were targeted by hsa-miR-214-3p and hsa-miR-2682-5p, and hsa-miR-214-5p and hsa-miR-199a-3p, respectively.
Numerous upregulated DEmiRs exhibited multiple predicted downregulated DEG targets, each with varying levels of prediction confidence. These prediction confidence levels, as determined by diverse sources within the IPA software, ranged from moderate to high and even extended to experimentally validated targets. For instance, the upregulated hsa-miR-371a-5p was experimentally confirmed to target STX1A, while both hsa-miR-145-5p and hsa-miR-10a-5p were experimentally validated to target KLF4. Additionally, hsa-miR-125b-1-3p was experimentally validated as a regulator of TNF. Moreover, 21 DEmiRs displayed high prediction levels for their target genes. Notably, the most upregulated hsa-miR-199a-5p was highly predicted to target IGFBP1. In this context, the miR-214 family, especially hsa-miR-214-3p, was found to target DLL1, HES6, LMX1B, MAFF, MAPK3, while hsa-miR-214-5p targeted NKX6.3, DLL4, SLC30A8, and STX1A. Furthermore, one significantly upregulated DEmiRs, hsa-miR-30b-3p, demonstrated regulatory influence on several critical genes, including NEUROG3, PAX4, MAFF, HES6, STX1A, SLC30A8, and PRKCG. In addition, hsa-miR-6815-5p, another significantly upregulated DEmiRs, exhibited a high number of DEG targets, encompassing LMX1B, MAPK3, HES6, IGFBP1, STX1A, and SLC30A8. Taken together, these findings strongly indicate the crucial involvement of the upregulated DEmiRs in regulating the expression of their target pancreatic genes and influencing the development and function of pancreatic islet cells. Nevertheless, it is essential to note that further experimental validation and functional studies are needed to solidify these predicted miRNA-mRNA networks.
Downregulated DEmiRs Potentially Target Upregulated Genes in FOXA2 –/– Islets
The cross-linked analysis between downregulated DEmiRs and upregulated DEGs in FOXA2–/– islets revealed 79 significantly downregulated DEmiRs (Log2 FC < − 1, p < 0.05), targeting 594 significantly upregulated DEGs (Log2 FC > 1, p < 0.05) in FOXA2–/– islets. Notably, nervous system development associated upregulated DEGs were found among the highly predicted targets of the downregulated DEmiRs. 55 DEmiRs (Log2 FC < − 1, p < 0.05) were found to be targeting 40 upregulated DEGs (Log2 FC > 2, p < 0.05) that are related to nervous system development (Fig. 5B and Supplementary Table 7). The most upregulated DEGs involved in this process, RELN, was predicted to be targeted by hsa-miR-429. Also, one of the most important DEGs for the neuron proliferation is LIN28A [38], which has been reported to ameliorate β-cell dysfunction and apoptosis [39]. LIN28A was targeted in our study by several DEmiRs such as hsa-let-7d-5p, hsa-miR-4510, hsa-miR-329-3p, hsa-miR-296-5p, and hsa-miR-181c-5p, which were downregulated in FOXA2–/– islets. We noticed the upregulation of EDNRB gene, which is associated with hypoxia in pancreatic islets [40]. Our study showed clear downregulation of miRNAs targeting EDNRB in the absence of FOXA2 such as hsa-miR-655-3p, hsa-miR-539-3p, hsa-miR-668-3p, hsa-miR-31-5p, and hsa-miR-340-5p. On the other hand, the same analysis revealed 34 significantly downregulated DEmiRs (Log2 FC < − 1, p < 0.05) targeting 27 significantly upregulated DEGs (Log2 FC > 1, p < 0.05) in FOXA2–/– islets associated with lipid and fat metabolism (Fig. 6B and Supplementary Table 8). For example, FOXA2 deletion was accompanied by downregulation in miRNAs targeting APOC3 such as hsa-miR-429 and hsa-miR-4728-5p, and APOC2 such as hsa-miR-4510 and hsa-miR-4728-5p. Upregulated PDGFRA/B were predicted targets of downregulated DEmiRs, including hsa-miR-654-5p, hsa-miR-892b, hsa-miR-146a-5p, and hsa-miR-301a-3p. Also, FOXA2 deletion led to the downregulation of miRNAs controlling the expression of the secretory phospholipase A2 (PLA2G2A and PLA2G12B), including hsa-miR-487a-5p, hsa-miR-4510, hsa-miR-4728-5p and hsa-miR-370-3p, hsa-miR-494-3p, hsa-miR-668-3p, and hsa-miR-892b.
Lack of FOXA2 alters transcriptomic and miRNA profiles related to Nervous system development. (A) Heatmap of upregulated DEGs associated with nervous system development in FOXA2–/– islets compared to WT-islets (n = 2). (B) Network reflecting target prediction analysis of downregulated DEmiRs and selected upregulated DEGs associated with nervous system development in FOXA2–/– islets. Green lines indicate experimentally observed targets, orange lines represent a high prediction confidence level, while grey lines indicate a moderate prediction confidence level
Loss of FOXA2 alters transcriptomic and miRNA profiles related to lipid metabolism. (A) Heatmap of upregulated DEGs associated with lipid metabolism in FOXA2–/– islets compared to WT-islets (n = 2). (B) Network reflecting target prediction analysis of downregulated DEmiRs and selected upregulated DEGs associated with lipid metabolism in FOXA2–/– islets. Green lines indicate experimentally observed targets, orange lines represent a high prediction confidence level, while grey lines indicate a moderate prediction confidence level
Discussion
Our recent study reported that FOXA2 deficiency significantly reduces the number of pancreatic INS- and GCG-producing cells [18]. In agreement with these findings, RNA-Seq analysis of FOXA2–/– islets has unveiled a substantial reduction in the expression of islet hormones and genes that play crucial roles in the development and function of pancreatic islet cells [2]. In this study, we identified several dysregulated miRNAs and mRNAs in pancreatic islets lacking FOXA2 and found that multiple DEmiRs were predicted to target several downregulated and upregulated DEGs. Conducting a pathway analysis on these target genes revealed an enrichment in biological processes, suggesting that miRNAs likely play significant roles in modulating pancreatic development and function. On the top list of enriched biological pathways affected by the downregulated DEGs in FOXA2–/– islets, pancreatic islet development, glucose hemostasis, and diabetes were significantly enriched.
We identified a total of 45 out of 61 significantly upregulated DEmiRs, which showed significant degrees of prediction for targeting 32 downregulated pancreatic DEGs. Many of these potential targets of miRNAs, which were significantly downregulated by FOXA2 loss are known to impact insulin synthesis and secretion and islet development. These key targets include PDX1, NKX6.1, PAX4, NKX6.3, INSM1, ARX, NEUROG3, NEUROD1, RFX6, MAFF, IGFBP1, TRPM4, PRKCG, SLC30A8, HES6, STX1A, DLL4, ADCY2, MAPK3, ALDH1A3, SHH, and IAPP. Previous studies demonstrated that IGFBP1 and TRPM4, playing critical roles in β-cell regeneration and insulin transport and secretion [41, 42], are predicted to be regulated by miR-199a-5p, which was the most upregulated miRNA in this study and is known to inhibit glucose metabolism [43]. Also, NKX6.3, DLL4, SLC30A8, and STX1A, which play key roles in insulin synthesis and release [33, 36, 44], are predicted to be targeted by miR-214-5p, which was significantly upregulated in FOXA2-deficient islets, and has been previously reported to be associated with type 1 diabetes (T1D) [45]. Furthermore, we found that IGFBP1, TRPM4, ADCY2, and MAFF were potential targets of miR-708-5p. It has been reported that increased miR-708 expression results in impaired insulin secretion and reduced proliferation of β-cells [9, 46]. Moreover, we observed a significant upregulation of miR-193b-3p, which was predicted to target PAX4, and PDX1. In addition, miR-193b-5p, was highly predicted to target RFX6, NKX6.1, MAFF, and MAPK3. Additionally, we observed an upregulation of miR-143-3p, which were predicted to target SLC30A8, MAPK3, and IAPP. Our study also identified several upregulated miRNAs associated with T1D, and T2D, including miR-372-3p [47], miR-100-5p [48], miR-152-5p [49], and miR-1269b [50]. In this study, miR-372-3p was predicted to target NEUROG3, NEUROD1, and ARX, while miR-100-5p was predicted to target INSM1, miR-152-5p targeted ALDH1A3 and TRPM4, and hsa-miR-1269b was predicted to target SHH, NEUROD1, SLC30A8, and MAFF.
Previous studies have reported that dysregulations in miRNA expression are implicated in β-cell dysfunction and the autoimmune destruction of pancreatic β-cells in T1D, while also affecting insulin sensitivity, and glucose metabolism in T2D [51]. Furthermore, altered miRNA expression patterns serve as potential biomarkers for predicting and diagnosing diabetes [52]. Our study revealed that FOXA2 deletion resulted in upregulation in several miRNAs, which are known to be elevated in the blood of T1D and T2D patients. Increased levels of miR-199a-5p [53, 54], miR-199a-3p [55, 56], miR-145-5p [57, 58], miR-145-3p [59], and miR-143-3p [60, 61] have been associated with both T1D and T2D. Also, miR-100-5p is dysregulated in both T1D & T1D. [48, 56]. In T1D patients, multiple miRNAs have been shown to be increased such as hsa-miR-1-3p [57], miR-214 family, miR-152 family [62], miR-10a-5p [48], miR-618 [63], and miR-365a-3p [64]. Previous studies have indicated elevated levels of several miRNAs in the blood and tissues of T2D patients, such as miR-122-5p, miR-105-5p, and miR-193b-3p [65, 66], miR-125b [67, 68], miR-34b-3p [69], miR-206, miR-371a-5p, miR-133a [70,71,72], miR-204-5p [73, 74], and miR-133b [74, 75]. Also, some of the DEmiRs are associated with diabetes complications, such as miR-133a-3p, which is associated with myocardial steatosis in T2D patients [76], and miR-129-2-3p [77] and miR-129-5p, which have been detected in T2D patients with macrovascular complications [78]. Furthermore, hsa-miR-155-5p is closely associated with diabetic macular edema (DME) [79]. Several upregulated miRNAs are detected to be associated with diabetic nephropathy (DN) patients such as, miR-372-3p [47], miR-548ah-3p, miR-760 [80], and miR-1269b [81]. Increased expression of miR-196b-5p observed in serum of patients with diabetic kidney disease [82]. The identified miRNAs in our study play pivotal roles in various aspects of diabetes pathogenesis, impacting β-cell biology and insulin resistance in insulin target tissues. Some miRNAs influence glucose transporters expression, with miR-143 and miR-133b modulate GLUT4 protein expression, and miR-193b-3p inhibiting GLUT2 expression [61, 65, 72]. Also, other miRNAs are involved in diabetes by modulating key TFs, such as miR-204-5p, which downregulates MafA expression, and miR-372-3p, which targets growth factor-16 [47, 74]. Moreover, miR-199a-5p and miR-204-5p induce apoptosis, ROS generation, and ER stress in human islets [54, 74]. Notably, hsa-miR-1-3p exhibits a significant increase and strong association with glycated hemoglobin levels in patients diagnosed with T1D [57]. In our study, all of these miRNAs associated with T1D and/or T2D were significantly upregulated. This suggests that the absence of FOXA2 leads to an increase in the expression of diabetes-associated miRNAs, which could serve as biomarkers for predicting and diagnosing diabetes.
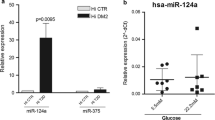
Although in this study we mainly focused on the downregulated DEGs associated with islet development and function, our findings demonstrated that several upregulated DEGs associated with nervous system development and lipid metabolism were predicted to be targeted by downregulated DEmiRs. This agrees with the previous study, which demonstrated that the deletion of Dicer, a critical factor in miRNA maturation, within PPs leads to significant impairments in the development of all islet cells, resulting in the upregulation of neuronal genes [83]. In our current study, we identified a significant downregulation of hsa-miR-375-3p in FOXA2–/– islets compared to WT controls. In human islets, the expression of miR-375 is increased during development [84], and its inactivation leads to diabetes and reduces the pancreatic islet cell mass [30, 85]. Collectively, these findings suggest that the absence of FOXA2 disrupts the differentiation of pancreatic islets, partially by elevating and suppressing the expression of multiple miRNAs, thereby exerting a biological influence on pancreatic islet development through regulated key developmental genes. In addition, the data propose that pancreatic development is intricately governed by a complex miRNA network that targets crucial pancreatic and non-pancreatic genes during the pancreatic islet differentiation. This network enhances signaling pathways required for proper islet development.
Conclusion
In conclusion, our findings demonstrate how the loss of FOXA2 disrupts the miRNA-mRNA regulatory network in iPSC-derived pancreatic islets, highlighting FOXA2’s crucial role in pancreatic endocrine cell generation and maturation. Our integration analysis revealed several dysregulated miRNAs targeting key pancreatic islet genes, shedding light on the intricate regulatory network controlling pancreatic development. While these insights are valuable, further validation studies are important to confirm the influence of miRNAs on pancreatic gene expression during islet differentiation. Moreover, deeper investigations into miRNA-mRNA interactions are essential for developing innovative diabetes therapeutic strategies.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Eizirik, D. L., Pasquali, L., & Cnop, M. (2020). Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nature Reviews. Endocrinology.
Al-Khawaga, S., et al. (2018). Pathways governing development of stem cell-derived pancreatic beta cells: Lessons from embryogenesis. Biological Reviews of the Cambridge Philosophical Society, 93(1), 364–389.
Benner, C., et al. (2014). The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. Bmc Genomics, 15, 620.
Baron, M., et al. (2016). A single-cell transcriptomic map of the human and mouse pancreas reveals Inter- and Intra-cell Population structure. Cell Syst, 3(4), 346–360e4.
Memon, B., & Abdelalim, E. M. (2022). Toward Precision Medicine with Human pluripotent stem cells for diabetes. Stem Cells Transl Med, 11(7), 704–714.
Abdelalim, E. M. (2021). Modeling different types of diabetes using human pluripotent stem cells. Cellular and Molecular Life Sciences, 78(6), 2459–2483.
Memon, B., & Abdelalim, E. M. (2020). Stem cell therapy for diabetes: Beta cells versus pancreatic progenitors. Cells, 9(2).
Eulalio, A., Huntzinger, E., & Izaurralde, E. (2008). Getting to the root of miRNA-mediated gene silencing. Cell, 132(1), 9–14.
Rodriguez-Comas, J., et al. (2017). Stress-Induced MicroRNA-708 impairs beta-cell function and growth. Diabetes, 66(12), 3029–3040.
Wang, W., et al. (2018). MiRNA-92a protects pancreatic B-cell function by targeting KLF2 in diabetes mellitus. Biochemical and Biophysical Research Communications, 500(3), 577–582.
Kanji, M. S., Martin, M. G., & Bhushan, A. (2013). Dicer1 is required to repress neuronal fate during endocrine cell maturation. Diabetes, 62(5), 1602–1611.
Lynn, F. C., et al. (2007). MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes, 56(12), 2938–2945.
Melkman-Zehavi, T., et al. (2011). miRNAs control insulin content in pancreatic beta-cells via downregulation of transcriptional repressors. Embo Journal, 30(5), 835–845.
Martinez-Sanchez, A., Nguyen-Tu, M. S., & Rutter, G. A. (2015). DICER inactivation identifies pancreatic beta-cell disallowed genes targeted by MicroRNAs. Molecular Endocrinology, 29(7), 1067–1079.
Martinez-Sanchez, A., Rutter, G. A., & Latreille, M. (2016). MiRNAs in beta-cell development, identity, and Disease. Frontiers in Genetics, 7, 226.
Kaspi, H., Pasvolsky, R., & Hornstein, E. (2014). Could microRNAs contribute to the maintenance of beta cell identity? Trends in Endocrinology and Metabolism, 25(6), 285–292.
Nesca, V., et al. (2013). Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia, 56(10), 2203–2212.
Elsayed, A. K., et al. (2021). Aberrant development of pancreatic beta cells derived from human iPSCs with FOXA2 deficiency. Cell Death & Disease, 12(1), 103.
Aghadi, M., Elgendy, R., & Abdelalim, E. M. (2022). Loss of FOXA2 induces ER stress and hepatic steatosis and alters developmental gene expression in human iPSC-derived hepatocytes. Cell Death and Disease, 13(8), 713.
Elsayed, A. K., Alajez, N. M., & Abdelalim, E. M. (2023). Genome-wide differential expression profiling of long non-coding RNAs in FOXA2 knockout iPSC-derived pancreatic cells. Cell Communication and Signaling, 21(1), 229.
Aldous, N., et al. (2023). iPSC-Derived pancreatic progenitors lacking FOXA2 reveal alterations in miRNA expression targeting key pancreatic genes. Stem Cell Reviews and Reports.
Vidigal, J. A., & Ventura, A. (2015). The biological functions of miRNAs: Lessons from in vivo studies. Trends in Cell Biology, 25(3), 137–147.
Ali, G., et al. (2020). Keratinocytes derived from patient-specific Induced pluripotent stem cells recapitulate the genetic signature of Psoriasis Disease. Stem Cells and Development, 29(7), 383–400.
Memon, B., et al. (2018). Enhanced differentiation of human pluripotent stem cells into pancreatic progenitors co-expressing PDX1 and NKX6.1 (Vol. 9, p. 15). Stem Cell Research & Therapy. 1.
Memon, B., & Abdelalim, E. M. (2022). Highly efficient differentiation of human pluripotent stem cells into pancreatic progenitors co-expressing PDX1 and NKX6.1. Methods in Molecular Biology, 2454, 351–363.
Shaath, H., & Elango, R. (2021). Alajez Molecular classification of breast Cancer utilizing long non-coding RNA (lncRNA) transcriptomes identifies Novel Diagnostic lncRNA Panel for Triple-negative breast Cancer. Cancers, 13. https://doi.org/10.3390/cancers13215350.
Huang, D. W., Sherman, B. T., & Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols, 4(1), 44–57.
Elango, R., et al. (2023). Transcriptome profiling and network enrichment analyses identify subtype-specific therapeutic gene targets for breast cancer and their microRNA regulatory networks. Cell Death and Disease, 14(7), 415.
LaPierre, M. P., & Stoffel, M. (2017). MicroRNAs as stress regulators in pancreatic beta cells and diabetes. Mol Metab, 6(9), 1010–1023.
Poy, M. N., et al. (2009). miR-375 maintains normal pancreatic α- and β-cell mass. Proceedings of the National Academy of Sciences, 106(14), 5813–5818.
Poy, M. N., et al. (2004). A pancreatic islet-specific microRNA regulates insulin secretion. Nature, 432(7014), 226–230.
Gauthier, B. R., & Wollheim, C. B. (2006). MicroRNAs: ‘ribo-regulators’ of glucose homeostasis. Nature Medicine, 12(1), 36–38.
Solomou, A., et al. (2015). The Zinc Transporter Slc30a8/ZnT8 is required in a subpopulation of pancreatic alpha-cells for hypoglycemia-induced Glucagon Secretion. Journal of Biological Chemistry, 290(35), 21432–21442.
Sui, L. (2023). ZnT8 loss of function mutation increases resistance of human embryonic stem cell-derived Beta cells to apoptosis in low Zinc Condition. Cells, 12(6).
Ma, L., et al. (2008). PCLO variants are nominally associated with early-onset type 2 diabetes and insulin resistance in Pima indians. Diabetes, 57(11), 3156–3160.
Liang, T., et al. (2017). New roles of Syntaxin-1A in insulin granule exocytosis and replenishment. Journal of Biological Chemistry, 292(6), 2203–2216.
Andersson, S. A., et al. (2012). Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes. Molecular and Cellular Endocrinology, 364(1–2), 36–45.
Wang, X. W., et al. (2018). Lin28 Signaling supports mammalian PNS and CNS Axon Regeneration. Cell Rep, 24(10), 2540–2552e6.
Hwang, Y. J., et al. (2021). Lin28a ameliorates glucotoxicity-induced beta-cell dysfunction and apoptosis. Bmb Reports, 54(4), 215–220.
Kugelmeier, P., et al. (2008). Expression and hypoxic regulation of the endothelin system in endocrine cells of human and rat pancreatic islets. JOP, 9(2), 133–149.
Lu, J., et al. (2016). IGFBP1 increases beta-cell regeneration by promoting alpha- to beta-cell transdifferentiation. Embo Journal, 35(18), 2026–2044.
Cheng, H., et al. (2007). TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium, 41(1), 51–61.
Xu, Y., et al. (2022). miRNA-199a-5p/SLC2A1 axis regulates glucose metabolism in non-small cell lung cancer. Journal of Cancer, 13(7), 2352–2361.
Rubey, M., et al. (2020). DLL1- and DLL4-Mediated Notch Signaling is essential for adult pancreatic islet homeostasis. Diabetes, 69(5), 915–926.
Grieco, G. E., et al. (2018). Serum levels of miR-148a and mir-21-5p are increased in type 1 Diabetic patients and correlated with markers of bone strength and metabolism. Non-Coding RNA, (4). https://doi.org/10.3390/ncrna4040037.
Jeffery, N., & Harries, L. W. (2016). beta-cell differentiation status in type 2 diabetes. Diabetes, Obesity & Metabolism, 18(12), 1167–1175.
Meng, Z., Li, F., & Wang, B. (2023). Mir-372-3p is a potential diagnostic factor for diabetic nephropathy and modulates high glucose-induced glomerular endothelial cell dysfunction via targeting fibroblast growth factor-16. Arch Med Sci, 19(3), 703–716.
Assmann, T. S., et al. (2017). MicroRNA expression profiles and type 1 diabetes mellitus: Systematic review and bioinformatic analysis. Endocr Connect, 6(8), 773–790.
Saleh, A. A., et al. (2022). Mi-RNA-93 and Mi-RNA-152 in the diagnosis of type 2 diabetes and Diabetic Retinopathy. British Journal of Biomedical Science, 79, 10192.
Prashanth, G., et al. (2021). Identification of hub genes related to the progression of type 1 diabetes by computational analysis. BMC Endocr Disord, 21(1), 61.
Hashimoto, N., & Tanaka, T. (2017). Role of miRNAs in the pathogenesis and susceptibility of diabetes mellitus. Journal of Human Genetics, 62(2), 141–150.
Bhatia, P., et al. (2015). miRNAs: Early prognostic biomarkers for type 2 diabetes mellitus? Biomarkers in Medicine, 9(10), 1025–1040.
Yang, L., et al. (2020). Hsa_circ_0060450 negatively regulates type I Interferon-Induced inflammation by serving as miR-199a-5p sponge in type 1 diabetes Mellitus. Frontiers in Immunology, 11, 576903.
Lin, N., et al. (2017). microRNA-199a-5p mediates high glucose-induced reactive oxygen species production and apoptosis in INS-1 pancreatic beta-cells by targeting SIRT1. European Review for Medical and Pharmacological Sciences, 21(5), 1091–1098.
Januszewski, A. S., et al. (2021). Insulin micro-secretion in type 1 diabetes and related microRNA profiles. Scientific Reports, 11(1), 11727.
Zhu, H., & Leung, S. W. (2023). MicroRNA biomarkers of type 2 diabetes: Evidence synthesis from meta-analyses and pathway modelling. Diabetologia, 66(2), 288–299.
Morales-Sanchez, P., et al. (2023). Circulating miRNA expression in long-standing type 1 diabetes mellitus. Scientific Reports, 13(1), 8611.
Nunez Lopez, Y. O., et al. (2019). Predicting and understanding the response to short-term intensive insulin therapy in people with early type 2 diabetes. Mol Metab, 20, 63–78.
Massaro, J. D., et al. (2019). Post-transcriptional markers associated with clinical complications in type 1 and type 2 diabetes mellitus. Molecular and Cellular Endocrinology, 490, 1–14.
Swolin-Eide, D., et al. (2023). Circulating microRNAs in young individuals with long-duration type 1 diabetes in comparison with healthy controls. Scientific Reports, 13(1), 11634.
Shrivastav, D., & Singh, D. D. (2024). Emerging roles of microRNAs as diagnostics and potential therapeutic interest in type 2 diabetes mellitus. World J Clin Cases, 12(3), 525–537.
Kim, M., & Zhang, X. (2019). The profiling and role of miRNAs in diabetes Mellitus. J Diabetes Clin Res, 1(1), 5–23.
Santos-Bezerra, D. P., et al. (2019). Micro-RNAs 518d-3p and 618 are upregulated in individuals with type 1 diabetes with multiple microvascular complications. Front Endocrinol (Lausanne), 10, 385.
Satake, E., et al. (2018). Circulating miRNA profiles Associated with hyperglycemia in patients with type 1 diabetes. Diabetes, 67(5), 1013–1023.
Hu, H., et al. (2022). Plasma miR-193b-3p is elevated in type 2 diabetes and could impair glucose metabolism. Front Endocrinol (Lausanne), 13, 814347.
Lee, H. M., et al. (2021). Detection of increased serum mir-122-5p and mir-455-3p levels before the clinical diagnosis of liver cancer in people with type 2 diabetes. Scientific Reports, 11(1), 23756.
de Candia, P., et al. (2017). A unique plasma microRNA profile defines type 2 diabetes progression. PLoS One, 12(12), e0188980.
Cheung, R., et al. (2022). Glucose-dependent miR-125b is a negative Regulator of beta-cell function. Diabetes, 71(7), 1525–1545.
Song, F., et al. (2021). MiR-34b-3p impaired HUVECs viability and Migration via Targeting PDK1 in an in vitro model of gestational diabetes Mellitus. Biochemical Genetics, 59(6), 1381–1395.
Fernandez-Valverde, S. L., Taft, R. J., & Mattick, J. S. (2011). MicroRNAs in beta-cell biology, insulin resistance, diabetes and its complications. Diabetes, 60(7), 1825–1831.
He, Y. (2017). A systematic study of dysregulated MicroRNA in type 2 diabetes Mellitus. International Journal of Molecular Sciences, 18(3).
Improta-Caria, A. C., et al. (2022). MicroRNAs in type 2 diabetes mellitus: Potential role of physical exercise. Reviews in Cardiovascular Medicine, 23(1), 29.
Zhu, X., et al. (2022). LncRNA LYPLAL1-DT screening from type 2 diabetes with macrovascular complication contributes protective effects on human umbilical vein endothelial cells via regulating the miR-204-5p/SIRT1 axis. Cell Death Discov, 8(1), 245.
Natalicchio, A., et al. (2023). MiRNA dysregulation underlying common pathways in type 2 diabetes and cancer development: An Italian Association of Medical Oncology (AIOM)/Italian Association of Medical Diabetologists (AMD)/Italian Society of Diabetology (SID)/Italian Society of Endocrinology (SIE)/Italian Society of Pharmacology (SIF) multidisciplinary critical view. ESMO Open, 8(3), 101573.
Zhong, H., et al. (2021). MicroRNA-133b inhibition restores EGFR expression and accelerates diabetes-impaired Wound Healing. Oxid Med Cell Longev, 2021, p9306760.
de Gonzalo-Calvo, D., et al. (2017). Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Scientific Reports, 7(1), 47.
Alamro, H., et al. (2022). Type 2 diabetes Mellitus and its comorbidity, Alzheimer’s disease: Identifying critical microRNA using machine learning. Front Endocrinol (Lausanne), 13, 1084656.
He, X. Y., & Ou, C. L. (2021). Clinical significance of serum mir-129-5p in patients with diabetes mellitus presenting macrovascular complications. World J Diabetes, 12(8), 1282–1291.
He, J., et al. (2021). Expression of microRNA-155-5p in patients with refractory diabetic macular edema and its regulatory mechanism. Exp Ther Med, 22(3), 975.
Lee, W. C. (2020). Urinary exosomal MicroRNA signatures in Nephrotic, Biopsy-Proven Diabetic Nephropathy. J Clin Med, 9(4).
Tsai, Y. C., et al. (2021). Autocrine Exosomal Fibulin-1 as a target of MiR-1269b induces epithelial-mesenchymal transition in Proximal Tubule in Diabetic Nephropathy. Front Cell Dev Biol, 9, 789716.
Hu, R. (2020). miR-196b-5p-enriched extracellular vesicles from tubular epithelial cells mediated aldosterone-induced renal fibrosis in mice with diabetes. BMJ Open Diabetes Res Care, 8(1).
Joglekar, M. V., Parekh, V. S., & Hardikar, A. A. (2007). New pancreas from old: Microregulators of pancreas regeneration. Trends in Endocrinology and Metabolism, 18(10), 393–400.
Joglekar, M. V., Joglekar, V. M., & Hardikar, A. A. (2009). Expression of islet-specific microRNAs during human pancreatic development. Gene Expression Patterns, 9(2), 109–113.
Latreille, M., et al. (2015). miR-375 gene dosage in pancreatic beta-cells: Implications for regulation of beta-cell mass and biomarker development. J Mol Med (Berl), 93(10), 1159–1169.
Acknowledgements
We thank the Genomic Core members at QBRI for their assistance for technical support in RNA and miRNA sequencing.
Funding
Open Access funding provided by the Qatar National Library. This work was funded by grants from Qatar Biomedical Research Institute (QBRI) (Grant No. IGP3 and QBRI-HSCI Project 1) and Sidra Medicine. The co-first author of this article, Noura Aldous, is a PhD student with a scholarship funded from QRDI (GSRA9-L-1-0511-22008).
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
A.K.E. and N.A. performed the experiments. A.K.E., N.A., and E.M.A. analyzed, interpreted the data, and wrote the manuscript. N.M.A. analyzed the RNA-Seq and miRNA-Seq data. All authors reviewed and approved the final version of the manuscript. E.M.A. conceived and designed the study and obtained research funding.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsayed, A.K., Aldous, N., Alajez, N.M. et al. Identifying miRNA Signatures Associated with Pancreatic Islet Dysfunction in a FOXA2-Deficient iPSC Model. Stem Cell Rev and Rep (2024). https://doi.org/10.1007/s12015-024-10752-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s12015-024-10752-0