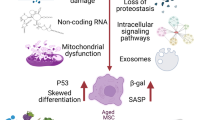
Abstract
Mesenchymal stem cells (MSCs) are extensively researched for therapeutic applications in tissue engineering and show significant potential for clinical use. Intrinsic or extrinsic factors causing senescence may lead to reduced proliferation, aberrant differentiation, weakened immunoregulation, and increased inflammation, ultimately limiting the potential of MSCs. It is crucial to comprehend the molecular pathways and internal processes responsible for the decline in MSC function due to senescence in order to devise innovative approaches for rejuvenating senescent MSCs and enhancing MSC treatment. We investigate the main molecular processes involved in senescence, aiming to provide a thorough understanding of senescence-related issues in MSCs. Additionally, we analyze the most recent advancements in cutting-edge approaches to combat MSC senescence based on current research. We are curious whether the aging process of stem cells results in a permanent “memory” and if cellular reprogramming may potentially revert the aging epigenome to a more youthful state.






Similar content being viewed by others
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
Maraldi, T., Guida, M., Zavatti, M., Resca, E., Bertoni, L., La Sala, G. B., & De Pol, A. (2015). Nuclear Nox4 Role in Stemness Power of Human Amniotic Fluid Stem Cells. Oxid Med Cell Longev 2015:101304.
Phinney, D. G., Galipeau, J., Krampera, M., Martin, I., Shi, Y., & Sensebe, L. (2013). MSCs: Science and trials. Nature Medicine, 19(7), 812.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D., & Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317.
Cui, J., Zhang, W., Huang, E., Wang, J., Liao, J., Li, R., Yu, X., Zhao, C., Zeng, Z., Shu, Y., et al. (2019). BMP9-induced osteoblastic differentiation requires functional notch signaling in mesenchymal stem cells. Laboratory Investigation, 99(1), 58–71.
He, Q., Wang, L., Zhao, R., Yan, F., Sha, S., Cui, C., Song, J., Hu, H., Guo, X., Yang, M., et al. (2020). Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Research & Therapy, 11(1), 223.
Hashemian, S. R., Aliannejad, R., Zarrabi, M., Soleimani, M., Vosough, M., Hosseini, S. E., Hossieni, H., Keshel, S. H., Naderpour, Z., Hajizadeh-Saffar, E., et al. (2021). Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: A case series. Stem Cell Research & Therapy, 12(1), 91.
Yin, J. Q., Zhu, J., & Ankrum, J. A. (2019). Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng, 3(2), 90–104.
Gorgoulis, V., Adams, P. D., Alimonti, A., Bennett, D. C., Bischof, O., Bishop, C., Campisi, J., Collado, M., Evangelou, K., Ferbeyre, G., et al. (2019). Cellular Senescence: Defining a path Forward. Cell, 179(4), 813–827.
Zhou, X., Hong, Y., Zhang, H., & Li, X. (2020). Mesenchymal stem cell senescence and rejuvenation: Current Status and challenges. Front Cell Dev Biol, 8, 364.
Khademi-Shirvan, M., Ghorbaninejad, M., Hosseini, S., & Baghaban Eslaminejad, M. (2020). The importance of Stem Cell Senescence in Regenerative Medicine. Advances in Experimental Medicine and Biology, 1288, 87–102.
Zhang, Q., Sun, X., Ding, J., He, P., Liu, Y., Cheng, H., Zhou, C., & Meng, X. (2015). Autoserum: An Optimal Supplement for Bone Marrow Mesenchymal Stem Cells of Liver-Injured Rats. Stem Cells Int 2015:459580.
Lepperdinger, G. (2011). Inflammation and mesenchymal stem cell aging. Current Opinion in Immunology, 23(4), 518–524.
Lei, Q., Gao, F., Liu, T., Ren, W., Chen, L., Cao, Y., Chen, W., Guo, S., Zhang, Q., Chen, W. (2021). Extracellular vesicles deposit PCNA to rejuvenate aged bone marrow-derived mesenchymal stem cells and slow age-related degeneration. Sci Transl Med 13(578).
Wu, Z., Zhang, W., Song, M., Wang, W., Wei, G., Li, W., Lei, J., Huang, Y., Sang, Y., Chan, P., et al. (2018). Differential stem cell aging kinetics in Hutchinson-Gilford progeria syndrome and Werner syndrome. Protein and Cell, 9(4), 333–350.
Liu, J., Ding, Y., Liu, Z., & Liang, X. (2020). Senescence in mesenchymal stem cells: Functional alterations, Molecular mechanisms, and rejuvenation strategies. Front Cell Dev Biol, 8, 258.
Cenni, V., Capanni, C., Mattioli, E., Schena, E., Squarzoni, S., Bacalini, M. G., Garagnani, P., Salvioli, S., Franceschi, C., & Lattanzi, G. (2020). Lamin a involvement in ageing processes. Ageing Research Reviews, 62, 101073.
Liang, S., Wang, F., Han, J., & Chen, K. (2020). Latent periodic process inference from single-cell RNA-seq data. Nature Communications, 11(1), 1441.
Hladik, D., Hofig, I., Oestreicher, U., Beckers, J., Matjanovski, M., Bao, X., Scherthan, H., Atkinson, M. J., & Rosemann, M. (2019). Long-term culture of mesenchymal stem cells impairs ATM-dependent recognition of DNA breaks and increases genetic instability. Stem Cell Research & Therapy, 10(1), 218.
Bassi, C., Fortin, J., Snow, B. E., Wakeham, A., Ho, J., Haight, J., You-Ten, A., Cianci, E., Buckler, L., Gorrini, C., et al. (2021). The PTEN and ATM axis controls the G1/S cell cycle checkpoint and tumorigenesis in HER2-positive breast cancer. Cell Death and Differentiation, 28(11), 3036–3051.
Wang, Y., Yang, L., Mao, L., Zhang, L., Zhu, Y., Xu, Y., Cheng, Y., Sun, R., Zhang, Y., Ke, J., et al. (2022). SGLT2 inhibition restrains thyroid cancer growth via G1/S phase transition arrest and apoptosis mediated by DNA damage response signaling pathways. Cancer Cell International, 22(1), 74.
Alessio, N., Del Gaudio, S., Capasso, S., Di Bernardo, G., Cappabianca, S., Cipollaro, M., Peluso, G., & Galderisi, U. (2015). Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget, 6(10), 8155–8166.
Nicolay, N. H., Liang, Y., Lopez Perez, R., Bostel, T., Trinh, T., Sisombath, S., Weber, K. J., Ho, A. D., Debus, J., Saffrich, R., et al. (2015). Mesenchymal stem cells are resistant to carbon ion radiotherapy. Oncotarget, 6(4), 2076–2087.
Cruet-Hennequart, S., Prendergast, A. M., Shaw, G., Barry, F. P., & Carty, M. P. (2012). Doxorubicin induces the DNA damage response in cultured human mesenchymal stem cells. International Journal of Hematology, 96(5), 649–656.
Liu, H., Huang, B., Xue, S., U, K. P., Tsang, L. L., Zhang, X., Li, G., & Jiang, X. (2020). Functional crosstalk between mTORC1/p70S6K pathway and heterochromatin organization in stress-induced senescence of MSCs. Stem Cell Research & Therapy, 11(1), 279.
Raz, V., Vermolen, B. J., Garini, Y., Onderwater, J. J., Mommaas-Kienhuis, M. A., Koster, A. J., Young, I. T., Tanke, H., & Dirks, R. W. (2008). The nuclear lamina promotes telomere aggregation and centromere peripheral localization during senescence of human mesenchymal stem cells. Journal of Cell Science, 121(Pt 24), 4018–4028.
Ge, Y., Wu, S., Xue, Y., Tao, J., Li, F., Chen, Y., Liu, H., Ma, W., Huang, J., & Zhao, Y. (2016). Preferential extension of short telomeres induced by low extracellular pH. Nucleic Acids Research, 44(17), 8086–8096.
Rajasingh, S., Sigamani, V., Selvam, V., Gurusamy, N., Kirankumar, S., Vasanthan, J., & Rajasingh, J. (2021). Comparative analysis of human induced pluripotent stem cell-derived mesenchymal stem cells and umbilical cord mesenchymal stem cells. Journal of Cellular and Molecular Medicine, 25(18), 8904–8919.
Aguado, J., di d’Adda, F., & Wolvetang, E. (2020). Telomere transcription in ageing. Ageing Research Reviews, 62, 101115.
Sharpless, N. E., & Sherr, C. J. (2015). Forging a signature of in vivo senescence. Nature Reviews Cancer, 15(7), 397–408.
Guillot, P. V., Gotherstrom, C., Chan, J., Kurata, H., & Fisk, N. M. (2007). Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells, 25(3), 646–654.
Tong, A. S., Stern, J. L., Sfeir, A., Kartawinata, M., de Lange, T., Zhu, X. D., & Bryan, T. M. (2015). ATM and ATR Signaling Regulate the Recruitment of Human Telomerase to telomeres. Cell Rep, 13(8), 1633–1646.
Lee, S. S., Bohrson, C., Pike, A. M., Wheelan, S. J., & Greider, C. W. (2015). ATM kinase is required for Telomere Elongation in Mouse and Human cells. Cell Rep, 13(8), 1623–1632.
Tchirkov, A., & Lansdorp, P. M. (2003). Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Human Molecular Genetics, 12(3), 227–232.
Zhang, Q., Cao, L., Zou, S., Feng, Y., Miao, X., Huang, L., & Wu, Y. (2022). Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying MicroRNA-181c-5p Promote BMP2-Induced Repair of Cartilage Injury through Inhibition of SMAD7 Expression. Stem Cells Int 2022:1157498.
Okada, M., Kim, H. W., Matsu-ura, K., Wang, Y. G., Xu, M., & Ashraf, M. (2016). Abrogation of Age-Induced MicroRNA-195 rejuvenates the senescent mesenchymal stem cells by reactivating telomerase. Stem Cells, 34(1), 148–159.
Pervaiz, S., Taneja, R., & Ghaffari, S. (2009). Oxidative stress regulation of stem and progenitor cells. Antioxidants & Redox Signaling, 11(11), 2777–2789.
Oh, J., Lee, Y. D., & Wagers, A. J. (2014). Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nature Medicine, 20(8), 870–880.
Korolchuk, V. I., Miwa, S., Carroll, B., & von Zglinicki, T. (2017). Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 21:7–13.
Ping, Z., Zhang, L. F., Cui, Y. J., Chang, Y. M., Jiang, C. W., Meng, Z. Z., Xu, P., Liu, H. Y., Wang, D. Y., & Cao, X. B. (2015). The Protective Effects of Salidroside from Exhaustive Exercise-Induced Heart Injury by Enhancing the PGC-1 alpha -NRF1/NRF2 Pathway and Mitochondrial Respiratory Function in Rats. Oxid Med Cell Longev 2015:876825.
Fang, E. F., Hou, Y., Palikaras, K., Adriaanse, B. A., Kerr, J. S., Yang, B., Lautrup, S., Hasan-Olive, M. M., Caponio, D., Dan, X., et al. (2019). Mitophagy inhibits amyloid-beta and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nature Neuroscience, 22(3), 401–412.
Zhang, F., Peng, W., Zhang, J., Dong, W., Wu, J., Wang, T., & Xie, Z. (2020). P53 and Parkin co-regulate mitophagy in bone marrow mesenchymal stem cells to promote the repair of early steroid-induced osteonecrosis of the femoral head. Cell Death and Disease, 11(1), 42.
Matsuda, N., & Tanaka, K. (2010). Uncovering the roles of PINK1 and parkin in mitophagy. Autophagy, 6(7), 952–954.
Checler, F., Goiran, T., & Alves da Costa, C. (2018). Nuclear TP53: An unraveled function as transcriptional repressor of PINK1. Autophagy, 14(6), 1099–1101.
Tsujimoto, T., Mori, T., Houri, K., Onodera, Y., Takehara, T., Shigi, K., Nakao, S., Teramura, T., & Fukuda, K. (2020). miR-155 inhibits mitophagy through suppression of BAG5, a partner protein of PINK1. Biochemical and Biophysical Research Communications, 523(3), 707–712.
Garcia-Prat, L., Martinez-Vicente, M., Perdiguero, E., Ortet, L., Rodriguez-Ubreva, J., Rebollo, E., Ruiz-Bonilla, V., Gutarra, S., Ballestar, E., Serrano, A. L., et al. (2016). Autophagy maintains stemness by preventing senescence. Nature, 529(7584), 37–42.
Guo, S. D., Yan, S. T., Li, W., Zhou, H., Yang, J. P., Yao, Y., Shen, M. J., Zhang, L. W., Zhang, H. B., & Sun, L. C. (2020). HDAC6 promotes sepsis development by impairing PHB1-mediated mitochondrial respiratory chain function. Aging (Albany NY), 12(6), 5411–5422.
Benameur, L., Charif, N., Li, Y., Stoltz, J. F., & de Isla, N. (2015). Toward an understanding of mechanism of aging-induced oxidative stress in human mesenchymal stem cells. Bio-medical Materials and Engineering, 25(1 Suppl), 41–46.
Burova, E., Borodkina, A., Shatrova, A., & Nikolsky, N. (2013). Sublethal oxidative stress induces the premature senescence of human mesenchymal stem cells derived from endometrium. Oxidative Medicine and Cellular Longevity, 2013, 474931.
Ko, E., Lee, K. Y., & Hwang, D. S. (2012). Human umbilical cord blood-derived mesenchymal stem cells undergo cellular senescence in response to oxidative stress. Stem Cells and Development, 21(11), 1877–1886.
Sahin, E., & Depinho, R. A. (2010). Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature, 464(7288), 520–528.
Jeong, S. G., & Cho, G. W. (2015). Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochemical and Biophysical Research Communications, 460(4), 971–976.
Passos, J. F., Saretzki, G., Ahmed, S., Nelson, G., Richter, T., Peters, H., Wappler, I., Birket, M. J., Harold, G., Schaeuble, K., et al. (2007). Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biology, 5(5), e110.
Passos, J. F., & von Zglinicki, T. (2005). Mitochondria, telomeres and cell senescence. Experimental Gerontology, 40(6), 466–472.
Garcia-Prat, L., Sousa-Victor, P., & Munoz-Canoves, P. (2017). Proteostatic and Metabolic Control of Stemness. Cell Stem Cell, 20(5), 593–608.
Vilchez, D., Boyer, L., Morantte, I., Lutz, M., Merkwirth, C., Joyce, D., Spencer, B., Page, L., Masliah, E., Berggren, W. T., et al. (2012). Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature, 489(7415), 304–308.
Aguilo, F., Zhang, F., Sancho, A., Fidalgo, M., Di Cecilia, S., Vashisht, A., Lee, D. F., Chen, C. H., Rengasamy, M., Andino, B., et al. (2015). Coordination of m(6)a mRNA methylation and gene transcription by ZFP217 regulates pluripotency and reprogramming. Cell Stem Cell, 17(6), 689–704.
You, K. T., Park, J., & Kim, V. N. (2015). Role of the small subunit processome in the maintenance of pluripotent stem cells. Genes & Development, 29(19), 2004–2009.
Artan, M., Hwang, A. B., Lee, S. V., & Nam, H. G. (2015). Meeting Report: International Symposium on the Genetics of Aging and Life History II. Aging (Albany NY) 7(6):362–369.
Ruz, C., Alcantud, J. L., Vives Montero, F., Duran, R., & Bandres-Ciga, S. (2020). Proteotoxicity and Neurodegenerative Diseases. International journal of molecular sciences 21(16).
Mizushima, N., & Komatsu, M. (2011). Autophagy: Renovation of cells and tissues. Cell, 147(4), 728–741.
Nixon, R. A. (2013). The role of autophagy in neurodegenerative disease. Nature Medicine, 19(8), 983–997.
Klionsky, D. J., Codogno, P., Cuervo, A. M., Deretic, V., Elazar, Z., Fueyo-Margareto, J., Gewirtz, D. A., Kroemer, G., Levine, B., Mizushima, N., et al. (2010). A comprehensive glossary of autophagy-related molecules and processes. Autophagy, 6(4), 438–448.
Vilchez, D., Simic, M. S., & Dillin, A. (2014). Proteostasis and aging of stem cells. Trends in Cell Biology, 24(3), 161–170.
Chichester, L., Wylie, A. T., Craft, S., & Kavanagh, K. (2015). Muscle heat shock protein 70 predicts insulin resistance with aging. The Journals of Gerontology Series A Biological Sciences and Medical Sciences, 70(2), 155–162.
Gottschling, D. E., & Nystrom, T. (2017). The upsides and downsides of Organelle Interconnectivity. Cell, 169(1), 24–34.
Goloubinoff, P. (2017). Editorial: The HSP70 Molecular Chaperone machines. Front Mol Biosci, 4, 1.
Drew, B. G., Ribas, V., Le, J. A., Henstridge, D. C., Phun, J., Zhou, Z., Soleymani, T., Daraei, P., Sitz, D., Vergnes, L., et al. (2014). HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes, 63(5), 1488–1505.
Lee, J. H., Yoon, Y. M., Song, K. H., Noh, H., & Lee, S. H. (2020). Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L-mitophagy pathway. Aging cell, 19(3), e13111.
Andersson, S., Romero, A., Rodrigues, J. I., Hua, S., Hao, X., Jacobson, T., Karl, V., Becker, N., Ashouri, A., Rauch, S. (2021). Genome-wide imaging screen uncovers molecular determinants of arsenite-induced protein aggregation and toxicity. Journal of Cell Science 134(11).
Ng, T. T., Mak, K. H., Popp, C., & Ng, R. K. (2020). Murine mesenchymal stromal cells retain biased differentiation plasticity towards their tissue of Origin. Cells 9(3).
Lan, Y. H., Pan, H., Li, C., Banks, K. M., Sam, J., Ding, B., Elemento, O., Goll, M. G., & Evans, T. (2019). TETs regulate Proepicardial Cell Migration through Extracellular Matrix Organization during Zebrafish Cardiogenesis. Cell Rep, 26(3), 720–.
Zhang, W., Qu, J., Liu, G. H., & Belmonte, J. C. I. (2020). The ageing epigenome and its rejuvenation. Nature Reviews Molecular cell Biology, 21(3), 137–150.
Torano, E. G., Bayon, G. F., Del Real, A., Sierra, M. I., Garcia, M. G., Carella, A., Belmonte, T., Urdinguio, R. G., Cubillo, I., Garcia-Castro, J., et al. (2016). Age-associated hydroxymethylation in human bone-marrow mesenchymal stem cells. J Transl Med, 14(1), 207.
Schellenberg, A., Lin, Q., Schuler, H., Koch, C. M., Joussen, S., Denecke, B., Walenda, G., Pallua, N., Suschek, C. V., Zenke, M., et al. (2011). Replicative senescence of mesenchymal stem cells causes DNA-methylation changes which correlate with repressive histone marks. Aging (Albany NY), 3(9), 873–888.
Franzen, J., Zirkel, A., Blake, J., Rath, B., Benes, V., Papantonis, A., & Wagner, W. (2017). Senescence-associated DNA methylation is stochastically acquired in subpopulations of mesenchymal stem cells. Aging Cell, 16(1), 183–191.
Pasumarthy, K. K., Doni Jayavelu, N., Kilpinen, L., Andrus, C., Battle, S. L., Korhonen, M., Lehenkari, P., Lund, R., Laitinen, S., & Hawkins, R. D. (2017). Methylome Analysis of Human Bone Marrow MSCs reveals extensive age- and Culture-Induced changes at Distal Regulatory Elements. Stem Cell Reports, 9(3), 999–1015.
Koch, C. M., Reck, K., Shao, K., Lin, Q., Joussen, S., Ziegler, P., Walenda, G., Drescher, W., Opalka, B., May, T., et al. (2013). Pluripotent stem cells escape from senescence-associated DNA methylation changes. Genome Research, 23(2), 248–259.
Zheng, Y., He, L., Wan, Y., & Song, J. (2013). H3K9me-enhanced DNA hypermethylation of the p16INK4a gene: An epigenetic signature for spontaneous transformation of rat mesenchymal stem cells. Stem Cells and Development, 22(2), 256–267.
Glaser, L. V., Steiger, M., Fuchs, A., van Bömmel, A., Einfeldt, E., Chung, H. R., Vingron, M., & Meijsing, S. H. (2021). Assessing genome-wide dynamic changes in enhancer activity during early mESC differentiation by FAIRE-STARR-seq. Nucleic Acids Research, 49(21), 12178–12195.
Chen, Y., Zhou, Y. L., Wu, H. W., Wang, L. S., Guo, R. Z., Yin, H. S., Wang, J., & Ai, S. Y. (2020). Photoelectrochemical assay for histone acetyltransferase based on polydopamine sensitized layered WS 2. Sensor Actuat B-Chem 319.
Jung, J. W., Lee, S., Seo, M. S., Park, S. B., Kurtz, A., Kang, S. K., & Kang, K. S. (2010). Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cellular and Molecular life Sciences: CMLS, 67(7), 1165–1176.
Li, Z., Liu, C., Xie, Z., Song, P., Zhao, R. C., Guo, L., Liu, Z., & Wu, Y. (2011). Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PloS One, 6(6), e20526.
Kukolj, T., Trivanovic, D., Mojsilovic, S., Djordjevic, I. O., Obradovic, H., Krstic, J., Jaukovic, A., & Bugarski, D. (2019). IL-33 guides osteogenesis and increases proliferation and pluripotency marker expression in dental stem cells. Cell Proliferat 52(1).
Khanban, H., Fattahi, E., & Talkhabi, M. (2019). In vivo administration of G9a inhibitor A366 decreases osteogenic potential of bone marrow-derived mesenchymal stem cells. Excli Journal, 18, 300–309.
Cakouros, D., Isenmann, S., Cooper, L., Zannettino, A., Anderson, P., Glackin, C., & Gronthos, S. (2012). Twist-1 induces Ezh2 recruitment regulating histone methylation along the Ink4A/Arf locus in mesenchymal stem cells. Molecular and Cellular Biology, 32(8), 1433–1441.
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P., & Brivanlou, A. H. (2004). Maintenance of pluripotency in human and mouse embryonic stem cells through activation of wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Medicine, 10(1), 55–63.
Lyashenko, N., Winter, M., Migliorini, D., Biechele, T., Moon, R. T., & Hartmann, C. (2011). Differential requirement for the dual functions of beta-catenin in embryonic stem cell self-renewal and germ layer formation. Nature Cell Biology, 13(7), 753–761.
Jeoung, J. Y., Nam, H. Y., Kwak, J., Jin, H. J., Lee, H. J., Lee, B. W., Baek, J. H., Eom, J. S., Chang, E. J., Shin, D. M., et al. (2015). A decline in Wnt3a signaling is necessary for mesenchymal stem cells to proceed to replicative senescence. Stem Cells and Development, 24(8), 973–982.
Zhao, L., Zhang, Y., Gao, Y., Geng, P., Lu, Y., Liu, X., Yao, R., Hou, P., Liu, D., Lu, J., et al. (2015). JMJD3 promotes SAHF formation in senescent WI38 cells by triggering an interplay between demethylation and phosphorylation of RB protein. Cell Death and Differentiation, 22(10), 1630–1640.
Tan, Y. N., Hu, Y. T., Xiao, Q., Tang, Y., Chen, H. Y., He, J. J., Chen, L. B., Jiang, K., Wang, Z. H., Yuan, Y., et al. (2020). Silencing of brain-expressed X-linked 2 (BEX2) promotes colorectal cancer metastasis through the hedgehog signaling pathway. International Journal of Biological Sciences, 16(2), 228–238.
Gu, Z., Tan, W., Feng, G., Meng, Y., Shen, B., Liu, H., & Cheng, C. (2014). Wnt/beta-catenin signaling mediates the senescence of bone marrow-mesenchymal stem cells from systemic lupus erythematosus patients through the p53/p21 pathway. Molecular and Cellular Biochemistry, 387(1–2), 27–37.
Zhang, D. Y., Pan, Y., Zhang, C., Yan, B. X., Yu, S. S., Wu, D. L., Shi, M. M., Shi, K., Cai, X. X., Zhou, S. S., et al. (2013). Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Molecular and Cellular Biochemistry, 374(1–2), 13–20.
Zhang, D. Y., Wang, H. J., & Tan, Y. Z. (2011). Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS One, 6(6), e21397.
Zhang, Y., Alexander, P. B., & Wang, X. F. (2017). TGF-beta Family Signaling in the control of cell proliferation and survival. Cold Spring Harbor Perspectives in Biology 9(4).
Debacq-Chainiaux, F., Borlon, C., Pascal, T., Royer, V., Eliaers, F., Ninane, N., Carrard, G., Friguet, B., de Longueville, F., Boffe, S., et al. (2005). Repeated exposure of human skin fibroblasts to UVB at subcytotoxic level triggers premature senescence through the TGF-beta1 signaling pathway. Journal of Cell Science, 118(Pt 4), 743–758.
Minagawa, S., Araya, J., Numata, T., Nojiri, S., Hara, H., Yumino, Y., Kawaishi, M., Odaka, M., Morikawa, T., Nishimura, S. L., et al. (2011). Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology, 300(3), L391–401.
Quere, R., Saint-Paul, L., Carmignac, V., Martin, R. Z., Chretien, M. L., Largeot, A., Hammann, A., Pais de Barros, J. P., Bastie, J. N., & Delva, L. (2014). Tif1gamma regulates the TGF-beta1 receptor and promotes physiological aging of hematopoietic stem cells. Proc Natl Acad Sci U S A, 111(29), 10592–10597.
Wu, J., Niu, J., Li, X., Wang, X., Guo, Z., & Zhang, F. (2014). TGF-beta1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. Bmc Developmental Biology, 14, 21.
Kawamura, H., Nakatsuka, R., Matsuoka, Y., Sumide, K., Fujioka, T., Asano, H., Iida, H., & Sonoda, Y. (2018). TGF-beta signaling accelerates senescence of human bone-derived CD271 and SSEA-4 double-positive mesenchymal stromal cells. Stem Cell Reports, 10(3), 920–932.
Jones, K. M., Saric, N., Russell, J. P., Andoniadou, C. L., Scambler, P. J., & Basson, M. A. (2015). CHD7 maintains neural stem cell quiescence and prevents premature stem cell depletion in the adult Hippocampus. Stem Cells, 33(1), 196–210.
Forte, E., Raja, A. N., Shamulailatpam, P., Manzano, M., Schipma, M. J., Casey, J. L., & Gottwein, E. (2015). MicroRNA-Mediated Transformation by the Kaposi’s Sarcoma-Associated Herpesvirus Kaposin Locus. Journal of Virology, 89(4), 2333–2341.
Tripathi, V., Sixt, K. M., Xu, X., & Zhang, Y. E. (2017). Direct regulation of alternative splicing by Smad3 through PCBP1 is essential to the tumor-promoting role of transforming growth factor beta1. Cancer Research 77.
Jo, E., Park, S. J., Choi, Y. S., Jeon, W. K., & Kim, B. C. (2015). Kaempferol suppresses transforming growth Factor-β1-Induced epithelial-to-mesenchymal transition and Migration of A549 Lung Cancer cells by inhibiting Akt1-Mediated phosphorylation of Smad3 at Threonine-179. Neoplasia (New York, N.Y.), 17(7), 525–537.
Jiang, W. J., Wang, X. Y., Su, S. W., Du, S., & Song, H. Q. (2022). Identifying the shared genes and KEGG pathways of Resolvin D1-targeted network and osteoarthritis using bioinformatics. Bioengineered, 13(4), 9839–9854.
Liu, F., Shi, J., Zhang, Y., Lian, A., Han, X., Zuo, K., Liu, M., Zheng, T., Zou, F., Liu, X. (2019). NANOG Attenuates Hair Follicle-Derived Mesenchymal Stem Cell Senescence by Upregulating PBX1 and Activating AKT Signaling. Oxidative medicine and cellular longevity 2019:4286213.
Liang, X., Ding, Y., Lin, F., Zhang, Y., Zhou, X., Meng, Q., Lu, X., Jiang, G., Zhu, H., Chen, Y., et al. (2019). Overexpression of ERBB4 rejuvenates aged mesenchymal stem cells and enhances angiogenesis via PI3K/AKT and MAPK/ERK pathways. The Faseb Journal, 33(3), 4559–4570.
Schaub, T., Gurgen, D., Maus, D., Lange, C., Tarabykin, V., Dragun, D., & Hegner, B. (2019). mTORC1 and mTORC2 differentially regulate cell Fate Programs to Coordinate Osteoblastic differentiation in mesenchymal stromal cells. Scientific Reports, 9(1), 20071.
Antonioli, E., Torres, N., Ferretti, M., Piccinato, C. A., & Sertie, A. L. (2019). Individual response to mTOR inhibition in delaying replicative senescence of mesenchymal stromal cells. PloS One, 14(1), e0204784.
Yang, M., Teng, S., Ma, C., Yu, Y., Wang, P., & Yi, C. (2018). Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology, 70(5), 1301–1313.
Zhang, D., Lu, H., Chen, Z., Wang, Y., Lin, J., Xu, S., Zhang, C., Wang, B., Yuan, Z., Feng, X., et al. (2017). High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Molecular Medicine Reports, 16(2), 1685–1690.
Gharibi, B., Farzadi, S., Ghuman, M., & Hughes, F. J. (2014). Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells, 32(8), 2256–2266.
Al-Azab, M., Wang, B., Elkhider, A., Walana, W., Li, W., Yuan, B., Ye, Y., Tang, Y., Almoiliqy, M., Adlat, S., et al. (2020). Indian hedgehog regulates senescence in bone marrow-derived mesenchymal stem cell through modulation of ROS/mTOR/4EBP1, p70S6K1/2 pathway. Aging (Albany Ny), 12(7), 5693–5715.
Coutu, D. L., Francois, M., & Galipeau, J. (2011). Inhibition of cellular senescence by developmentally regulated FGF receptors in mesenchymal stem cells. Blood, 117(25), 6801–6812.
Ito, T., Sawada, R., Fujiwara, Y., Seyama, Y., & Tsuchiya, T. (2007). FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochemical and Biophysical Research Communications, 359(1), 108–114.
Zhang, J. M., Feng, F. E., Wang, Q. M., Zhu, X. L., Fu, H. X., Xu, L. P., Liu, K. Y., Huang, X. J., & Zhang, X. H. (2016). Platelet-derived growth Factor-BB protects mesenchymal stem cells (MSCs) derived from Immune Thrombocytopenia patients against apoptosis and senescence and maintains MSC-Mediated immunosuppression. Stem Cells Transl Med, 5(12), 1631–1643.
Song, H. F., He, S., Li, S. H., Yin, W. J., Wu, J., Guo, J., Shao, Z. B., Zhai, X. Y., Gong, H., Lu, L., et al. (2017). Aged human multipotent mesenchymal stromal cells can be rejuvenated by neuron-derived neurotrophic factor and improve heart function after Injury. JACC Basic to Translational Science, 2(6), 702–716.
Chen, C. Y., Tseng, K. Y., Lai, Y. L., Chen, Y. S., Lin, F. H., & Lin, S. (2017). Overexpression of insulin-like Growth factor 1 enhanced the osteogenic capability of aging bone marrow mesenchymal stem cells. Theranostics, 7(6), 1598–1611.
Zhang, D., Yan, B., Yu, S., Zhang, C., Wang, B., Wang, Y., Wang, J., Yuan, Z., Zhang, L., & Pan, J. (2015). Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxidative medicine and cellular longevity 2015:867293.
Cao, Y., Yang, T., Gu, C., & Yi, D. (2013). Pigment epithelium-derived factor delays cellular senescence of human mesenchymal stem cells in vitro by reducing oxidative stress. Cell Biology International, 37(4), 305–313.
Xia, W., & Hou, M. (2018). Macrophage migration inhibitory factor rescues mesenchymal stem cells from doxorubicin-induced senescence though the PI3K-Akt signaling pathway. International Journal of Molecular Medicine, 41(2), 1127–1137.
Park, S. Y., Jeong, A. J., Kim, G. Y., Jo, A., Lee, J. E., Leem, S. H., Yoon, J. H., Ye, S. K., & Chung, J. W. (2017). Lactoferrin protects human mesenchymal stem cells from oxidative stress-Induced Senescence and apoptosis. Journal of Microbiology and Biotechnology, 27(10), 1877–1884.
Bahmani, B., Roudkenar, M. H., Halabian, R., Jahanian-Najafabadi, A., Amiri, F., & Jalili, M. A. (2014). Lipocalin 2 decreases senescence of bone marrow-derived mesenchymal stem cells under sub-lethal doses of oxidative stress. Cell Stress & Chaperones, 19(5), 685–693.
Alessio, N., Stellavato, A., Squillaro, T., Del Gaudio, S., Di Bernardo, G., Peluso, G., De Rosa, M., Schiraldi, C., & Galderisi, U. (2018). Hybrid complexes of high and low molecular weight hyaluronan delay in vitro replicative senescence of mesenchymal stromal cells: A pilot study for future therapeutic application. Aging (Albany Ny), 10(7), 1575–1585.
Wu, G., Xu, R., Zhang, P., Xiao, T., Fu, Y., Zhang, Y., Du, Y., Ye, J., Cheng, J., & Jiang, H. (2018). Estrogen regulates stemness and senescence of bone marrow stromal cells to prevent osteoporosis via ERbeta-SATB2 pathway. Journal of Cellular Physiology, 233(5), 4194–4204.
Breu, A., Sprinzing, B., Merkl, K., Bechmann, V., Kujat, R., Jenei-Lanzl, Z., Prantl, L., & Angele, P. (2011). Estrogen reduces cellular aging in human mesenchymal stem cells and chondrocytes. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 29(10), 1563–1571.
Zhang, M., Du, Y., Lu, R., Shu, Y., Zhao, W., Li, Z., Zhang, Y., Liu, R., Yang, T., Luo, S. (2016). Cholesterol Retards Senescence in Bone Marrow Mesenchymal Stem Cells by Modulating Autophagy and ROS/p53/p21(Cip1/Waf1) Pathway. Oxidative medicine and cellular longevity 2016:7524308.
Zhou, T., Yan, Y., Zhao, C., Xu, Y., Wang, Q., & Xu, N. (2019). Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Report: Communications in free Radical Research, 24(1), 62–69.
Lv, Y. J., Yang, Y., Sui, B. D., Hu, C. H., Zhao, P., Liao, L., Chen, J., Zhang, L. Q., Yang, T. T., Zhang, S. F., et al. (2018). Resveratrol counteracts bone loss via mitofilin-mediated osteogenic improvement of mesenchymal stem cells in senescence-accelerated mice. Theranostics, 8(9), 2387–2406.
Zhou, L., Chen, X., Liu, T., Gong, Y., Chen, S., Pan, G., Cui, W., Luo, Z. P., Pei, M., Yang, H., et al. (2015). Melatonin reverses H2 O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. Journal of Pineal Research, 59(2), 190–205.
Yang, F., Yang, L., Li, Y., Yan, G., Feng, C., Liu, T., Gong, R., Yuan, Y., Wang, N., Idiiatullina, E. (2017). Melatonin protects bone marrow mesenchymal stem cells against iron overload-induced aberrant differentiation and senescence. Journal of Pineal Research 63(3).
Shuai, Y., Liao, L., Su, X., Yu, Y., Shao, B., Jing, H., Zhang, X., Deng, Z., & Jin, Y. (2016). Melatonin Treatment improves mesenchymal stem cells therapy by preserving stemness during long-term in Vitro Expansion. Theranostics, 6(11), 1899–1917.
Li, C., Wei, G. J., Xu, L., Rong, J. S., Tao, S. Q., & Wang, Y. S. (2017). The involvement of senescence induced by the telomere shortness in the decline of osteogenic differentiation in BMSCs. European Review for Medical and Pharmacological Sciences, 21(5), 1117–1124.
Chen, H., Shi, B., Feng, X., Kong, W., Chen, W., Geng, L., Chen, J., Liu, R., Li, X., Chen, W., et al. (2015). Leptin and neutrophil-activating peptide 2 promote mesenchymal stem cell senescence through activation of the phosphatidylinositol 3-Kinase/Akt pathway in patients with systemic Lupus Erythematosus. Arthritis & Rheumatology (Hoboken NJ), 67(9), 2383–2393.
Gurung, S., Williams, S., Deane, J. A., Werkmeister, J. A., & Gargett, C. E. (2018). The transcriptome of human endometrial mesenchymal stem cells under TGFbetaR inhibition reveals improved potential for cell-based therapies. Front Cell Dev Biol, 6, 164.
Khorraminejad-Shirazi, M., Sani, M., Talaei-Khozani, T., Dorvash, M., Mirzaei, M., Faghihi, M. A., Monabati, A., & Attar, A. (2020). AICAR and nicotinamide treatment synergistically augment the proliferation and attenuate senescence-associated changes in mesenchymal stromal cells. Stem Cell Research & Therapy, 11(1), 45.
Li, Y., Zhang, W., Chang, L., Han, Y., Sun, L., Gong, X., Tang, H., Liu, Z., Deng, H., Ye, Y., et al. (2016). Vitamin C alleviates aging defects in a stem cell model for Werner syndrome. Protein and Cell, 7(7), 478–488.
Yan, X., Ehnert, S., Culmes, M., Bachmann, A., Seeliger, C., Schyschka, L., Wang, Z., Rahmanian-Schwarz, A., Stockle, U., De Sousa, P. A., et al. (2014). 5-azacytidine improves the osteogenic differentiation potential of aged human adipose-derived mesenchymal stem cells by DNA demethylation. PloS One, 9(6), e90846.
Marycz, K., Houston, J. M. I., Weiss, C., Rocken, M., & Kornicka, K. (2019). 5-Azacytidine and Resveratrol Enhance Chondrogenic Differentiation of Metabolic Syndrome-Derived Mesenchymal Stem Cells by Modulating Autophagy. Oxidative medicine and cellular longevity 2019:1523140.
Chang, T. C., Hsu, M. F., Shih, C. Y., & Wu, K. K. (2017). 5-methoxytryptophan protects MSCs from stress induced premature senescence by upregulating FoxO3a and mTOR. Scientific Reports, 7(1), 11133.
Klotz, B., Mentrup, B., Regensburger, M., Zeck, S., Schneidereit, J., Schupp, N., Linden, C., Merz, C., Ebert, R., & Jakob, F. (2012). 1,25-dihydroxyvitamin D3 treatment delays cellular aging in human mesenchymal stem cells while maintaining their multipotent capacity. PloS One, 7(1), e29959.
Ji, J., Fu, T., Dong, C., Zhu, W., Yang, J., Kong, X., Zhang, Z., Bao, Y., Zhao, R., Ge, X., et al. (2019). Targeting HMGB1 by ethyl pyruvate ameliorates systemic lupus erythematosus and reverses the senescent phenotype of bone marrow-mesenchymal stem cells. Aging (Albany Ny), 11(13), 4338–4353.
Wei, N., Yu, Y., Joshi, V., Schmidt, T., Qian, F., Salem, A. K., Stanford, C., & Hong, L. (2013). Glucocorticoid receptor antagonist and siRNA prevent senescence of human bone marrow mesenchymal stromal cells in vitro. Cell and Tissue Research, 354(2), 461–470.
Xu, Y., Yuan, H., Luo, Y., Zhao, Y. J., & Xiao, J. H. (2020). Ganoderic Acid D Protects Human Amniotic Mesenchymal Stem Cells against Oxidative Stress-Induced Senescence through the PERK/NRF2 Signaling Pathway. Oxidative medicine and cellular longevity 2020:8291413.
Strong, A. L., Jones, R. B. Jr., Glowacki, J., Boue, S. M., Burow, M. E., & Bunnell, B. A. (2017). Glycinol enhances osteogenic differentiation and attenuates the effects of age on mesenchymal stem cells. Regenerative Medicine, 12(5), 513–524.
Wang, Z., Jiang, R., Wang, L., Chen, X., Xiang, Y., Chen, L., Xiao, M., Ling, L., & Wang, Y. (2020). Ginsenoside Rg1 Improves Differentiation by Inhibiting Senescence of Human Bone Marrow Mesenchymal Stem Cell via GSK-3beta and beta-Catenin. Stem Cells Int 2020:2365814.
Wang, K., Su, Y., Liang, Y. T., Song, Y. H., & Wang, L. P. (2019). Oral DhHP-6 for the treatment of type 2 diabetes Mellitus. International Journal of Molecular Sciences 20(6).
Geng, L., Liu, Z., Zhang, W., Li, W., Wu, Z., Wang, W., Ren, R., Su, Y., Wang, P., Sun, L., et al. (2019). Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein and Cell, 10(6), 417–435.
Zhang, D., Yu, K., Yang, J., Xie, S., Yang, J., & Tan, L. (2020). Senolytic controls bone marrow mesenchymal stem cells fate improving bone formation. Am J Transl Res, 12(6), 3078–3088.
Suvakov, S., Cubro, H., White, W. M., Butler Tobah, Y. S., Weissgerber, T. L., Jordan, K. L., Zhu, X. Y., Woollard, J. R., Chebib, F. T., Milic, N. M., et al. (2019). Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biology of sex Differences, 10(1), 49.
Chen, G., Zhang, Y., Yu, S., Sun, W., & Miao, D. (2019). Bmi1 overexpression in mesenchymal stem cells exerts Antiaging and Antiosteoporosis effects by inactivating p16/p19 signaling and inhibiting oxidative stress. Stem Cells, 37(9), 1200–1211.
Son, S., Liang, M. S., Lei, P., Xue, X., Furlani, E. P., & Andreadis, S. T. (2015). Magnetofection mediated transient NANOG overexpression enhances proliferation and myogenic differentiation of human hair follicle derived mesenchymal stem cells. Bioconjugate Chemistry, 26(7), 1314–1327.
Li, H., Liu, P., Xu, S., Li, Y., Dekker, J. D., Li, B., Fan, Y., Zhang, Z., Hong, Y., Yang, G., et al. (2017). FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. The Journal of Clinical Investigation, 127(4), 1241–1253.
Fu, L., Hu, Y., Song, M., Liu, Z., Zhang, W., Yu, F. X., Wu, J., Wang, S., Izpisua Belmonte, J. C., Chan, P., et al. (2019). Up-regulation of FOXD1 by YAP alleviates senescence and osteoarthritis. PLoS Biology, 17(4), e3000201.
Li, Z. G., Li, J., Kong, Y. F., Yan, S., Ahmad, N., & Liu, X. Q. (2017). Plk1 phosphorylation of Mre11 antagonizes the DNA damage response. Cancer Research, 77(12), 3169–3180.
Zhang, Y. L., Zhu, W. W., He, H. W., Fan, B. H., Deng, R., Hong, Y. M., Liang, X. T., Zhao, H. Y., Li, X., & Zhang, F. X. (2019). Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging-Us, 11(24), 12641–12660.
Chen, X. Y., Wang, Q., Gu, K., Li, A. N., Fu, X. C., Wang, Y., Gu, W. T., & Wen, Y. (2019). Effect of YAP on an Immortalized Periodontal Ligament Stem Cell Line. Stem Cells International 2019.
Mazzoni, E., D’Agostino, A., Iaquinta, M. R., Bononi, I., Trevisiol, L., Rotondo, J. C., Patergnani, S., Giorgi, C., Gunson, M. J., Arnett, G. W., et al. (2020). Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem Cells Transl Med, 9(3), 377–388.
Hayashida, K., Sano, M., Ohsawa, I., Shinmura, K., Tamaki, K., Kimura, K., Endo, J., Katayama, T., Kawamura, A., Kohsaka, S., et al. (2008). Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochemical and Biophysical Research Communications, 373(1), 30–35.
Cardinal, J. S., Zhan, J., Wang, Y., Sugimoto, R., Tsung, A., McCurry, K. R., Billiar, T. R., & Nakao, A. (2010). Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney International, 77(2), 101–109.
Klichko, V. I., Safonov, V. L., Safonov, M. Y., & Radyuk, S. N. (2019). Supplementation with hydrogen-producing composition confers beneficial effects on physiology and life span in Drosophila. Heliyon, 5(5), e01679.
Sakai, T., Kurokawa, R., Hirano, S. I., & Imai, J. (2019). Hydrogen indirectly suppresses increases in hydrogen peroxide in cytoplasmic Hydroxyl Radical-Induced cells and suppresses Cellular Senescence. International Journal of Molecular Sciences 20(2).
Sakai, T., Imai, J., Takagaki, H., Ui, M., & Hatta, S. (2019). Cytoplasmic OH scavenger TA293 attenuates cellular senescence and fibrosis by activating macrophages through oxidized phospholipids/TLR4. Life Sciences, 221, 284–292.
Paduszynski, P., Aleksander-Konert, E., Zajdel, A., Wilczok, A., Jelonek, K., Witek, A., & Dzierzewicz, Z. (2016). Changes in expression of cartilaginous genes during chondrogenesis of Wharton’s jelly mesenchymal stem cells on three-dimensional biodegradable poly(L-lactide-co-glycolide) scaffolds. Cellular & Molecular Biology Letters, 21, 14.
Zhang, W., Huang, C., Sun, A., Qiao, L., Zhang, X., Huang, J., Sun, X., Yang, X., & Sun, S. (2018). Hydrogen alleviates cellular senescence via regulation of ROS/p53/p21 pathway in bone marrow-derived mesenchymal stem cells in vivo. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 106, 1126–1134.
Li, P., Lan, W., Li, J., Zhang, Y., Xiong, Q., Ye, J., Wu, C., & Xiao, H. (2022). Identification and functional evaluation of a Novel TBX4 mutation underlies small Patella syndrome. International Journal of Molecular Sciences 23(4).
Gao, S., Xiang, C., Qin, K., & Sun, C. (2018). Mathematical Modeling Reveals the Role of Hypoxia in the Promotion of Human Mesenchymal Stem Cell Long-Term Expansion. Stem Cells Int 2018:9283432.
Kim, D. S., Ko, Y. J., Lee, M. W., Park, H. J., Park, Y. J., Kim, D. I., Sung, K. W., Koo, H. H., & Yoo, K. H. (2016). Effect of low oxygen tension on the biological characteristics of human bone marrow mesenchymal stem cells. Cell Stress & Chaperones, 21(6), 1089–1099.
Abdulkina, L. R., Kobayashi, C., Lovell, J. T., Chastukhina, I. B., Aklilu, B. B., Agabekian, I. A., Suescun, A. V., Valeeva, L. R., Nyamsuren, C., Aglyamova, G. V., et al. (2019). Components of the ribosome biogenesis pathway underlie establishment of telomere length set point in Arabidopsis. Nature Communications, 10(1), 5479.
Waseem, M., Khan, I., Iqbal, H., Eijaz, S., Usman, S., Ahmed, N., Alam, G., & Salim, A. (2016). Hypoxic preconditioning improves the therapeutic potential of aging bone marrow mesenchymal stem cells in Streptozotocin-Induced Type-1 Diabetic mice. Cellular Reprogramming, 18(5), 344–355.
Tsai, C. C., Chen, Y. J., Yew, T. L., Chen, L. L., Wang, J. Y., Chiu, C. H., & Hung, S. C. (2011). Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood, 117(2), 459–469.
Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science 367(6478).
Jeppesen, D. K., Fenix, A. M., Franklin, J. L., Higginbotham, J. N., Zhang, Q., Zimmerman, L. J., Liebler, D. C., Ping, J., Liu, Q., Evans, R., et al. (2019). Reassessment of Exosome Composition. Cell, 177(2), 428–445e418.
van Niel, G., D’Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 19(4), 213–228.
Eitan, E., Green, J., Bodogai, M., Mode, N. A., Baek, R., Jorgensen, M. M., Freeman, D. W., Witwer, K. W., Zonderman, A. B., Biragyn, A., et al. (2017). Age-related changes in plasma extracellular vesicle characteristics and internalization by Leukocytes. Scientific Reports, 7(1), 1342.
Yu, L., Wen, H., Liu, C., Wang, C., Yu, H., Zhang, K., Han, Q., Liu, Y., Han, Z., Li, Z., et al. (2023). Embryonic stem cell-derived extracellular vesicles rejuvenate senescent cells and antagonize aging in mice. Bioact Mater, 29, 85–97.
Hu, G., Xia, Y., Zhang, J., Chen, Y., Yuan, J., Niu, X., Zhao, B., Li, Q., Wang, Y., & Deng, Z. (2020). ESC-sEVs rejuvenate senescent hippocampal NSCs by activating lysosomes to improve cognitive dysfunction in vascular dementia. Adv Sci (Weinh), 7(10), 1903330.
Oh, M., Lee, J., Kim, Y. J., Rhee, W. J., & Park, J. H. (2018). Exosomes Derived from Human Induced Pluripotent stem cells ameliorate the aging of skin fibroblasts. International Journal of Molecular Sciences 19(6).
Liu, S., Mahairaki, V., Bai, H., Ding, Z., Li, J., Witwer, K. W., & Cheng, L. (2019). Highly purified human extracellular vesicles produced by stem cells alleviate Aging Cellular phenotypes of senescent human cells. Stem Cells, 37(6), 779–790.
Sahu, A., Clemens, Z. J., Shinde, S. N., Sivakumar, S., Pius, A., Bhatia, A., Picciolini, S., Carlomagno, C., Gualerzi, A., Bedoni, M., et al. (2021). Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nat Aging, 1(12), 1148–1161.
Antoni, D., Burckel, H., Josset, E., & Noel, G. (2015). Three-dimensional cell culture: A breakthrough in vivo. International Journal of Molecular Sciences, 16(3), 5517–5527.
Lee, Y. W., Fu, S. C., Yeung, M. Y., Lau, C. M. L., Chan, K. M., & Hung, L. K. (2017). Effects of Redox Modulation on Cell Proliferation, viability, and Migration in cultured rat and human tendon progenitor cells. Oxid Med Cell Longev, 2017, 8785042.
Rustad, K. C., Wong, V. W., Sorkin, M., Glotzbach, J. P., Major, M. R., Rajadas, J., Longaker, M. T., & Gurtner, G. C. (2012). Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials, 33(1), 80–90.
Garg, R. K., Rennert, R. C., Duscher, D., Sorkin, M., Kosaraju, R., Auerbach, L. J., Lennon, J., Chung, M. T., Paik, K., Nimpf, J., et al. (2014). Capillary force seeding of hydrogels for adipose-derived stem cell delivery in wounds. Stem Cells Transl Med, 3(9), 1079–1089.
Cheng, N. C., Chen, S. Y., Li, J. R., & Young, T. H. (2013). Short-term spheroid formation enhances the regenerative capacity of adipose-derived stem cells by promoting stemness, angiogenesis, and chemotaxis. Stem Cells Transl Med, 2(8), 584–594.
Tseng, T. C., Wong, C. W., Hsieh, F. Y., & Hsu, S. H. (2017). Biomaterial substrate-mediated multicellular spheroid formation and their applications in tissue Engineering. Biotechnology Journal 12(12).
Gionet-Gonzales, M., Casella, A., Diloretto, D., Ginnell, C., Griffin, K. H., Bigot, A., & Leach, J. K. (2021). Sulfated Alginate Hydrogels prolong the therapeutic potential of MSC spheroids by sequestering the Secretome. Adv Healthc Mater, 10(21), e2101048.
Shen, H., Ding, C., Yuan, S., Pan, T., Li, D., Li, H., Huang, B., & Liu, Q. (2020). Vitamin C- and Valproic Acid-Induced fetal RPE stem-like cells recover retinal degeneration via regulating SOX2. Molecular Therapy, 28(7), 1645–1657.
Ha, J., Kim, B. S., Min, B., Nam, J., Lee, J. G., Lee, M., Yoon, B. H., Choi, Y. H., Im, I., Park, J. S., et al. (2022). Intermediate cells of in vitro cellular reprogramming and in vivo tissue regeneration require desmoplakin. Science Advances, 8(43), eabk1239.
Rando, T. A., & Chang, H. Y. (2012). Aging, rejuvenation, and epigenetic reprogramming: Resetting the aging clock. Cell, 148(1–2), 46–57.
Domingues, S., Darle, A., Masson, Y., Saidani, M., Lagoutte, E., Bejanariu, A., Coutier, J., Ayata, R. E., Bouschbacher, M., Peschanski, M. (2022). Clinical Grade Human Pluripotent Stem Cell-Derived Engineered Skin Substitutes Promote Keratinocytes Wound Closure In Vitro. Cells 11(7).
Victor, M. B., Richner, M., Olsen, H. E., Lee, S. W., Monteys, A. M., Ma, C., Huh, C. J., Zhang, B., Davidson, B. L., Yang, X. W., et al. (2020). Author correction: Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes. Nature Neuroscience, 23(10), 1307.
Wang, S., Liu, Z., Ye, Y., Li, B., Liu, T., Zhang, W., Liu, G. H., Zhang, Y. A., Qu, J., Xu, D., et al. (2018). Ectopic hTERT expression facilitates reprograming of fibroblasts derived from patients with Werner syndrome as a WS cellular model. Cell Death and Disease, 9(9), 923.
Shao, K., Koch, C., Gupta, M. K., Lin, Q., Lenz, M., Laufs, S., Denecke, B., Schmidt, M., Linke, M., Hennies, H. C., et al. (2013). Induced pluripotent mesenchymal stromal cell clones retain donor-derived differences in DNA methylation profiles. Molecular Therapy: The Journal of the American Society of Gene Therapy, 21(1), 240–250.
Frobel, J., Hemeda, H., Lenz, M., Abagnale, G., Joussen, S., Denecke, B., Saric, T., Zenke, M., & Wagner, W. (2014). Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem cell Reports, 3(3), 414–422.
Kim, M., Bae, Y. K., Um, S., Kwon, J. H., Kim, G. H., Choi, S. J., Oh, W., & Jin, H. J. (2020). A Small-Sized Population of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Shows High Stemness Properties and Therapeutic Benefit. Stem Cells Int 2020:5924983.
Hou, P., Li, Y., Zhang, X., Liu, C., Guan, J., Li, H., Zhao, T., Ye, J., Yang, W., Liu, K., et al. (2013). Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science, 341(6146), 651–654.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Hongqing Zhao: Conceptualization, Investigation, Writing - original draft. Houming Zhao: Methodology, Draw. Shuaifei Ji: Supervision, Writing - original draft and revision. All of the authors have read and approved the final manuscript. Hongqing Zhao and Houming Zhao contributed to this work equally.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
The authors declare that there is no confict of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, H., Zhao, H. & Ji, S. A Mesenchymal stem cell Aging Framework, from Mechanisms to Strategies. Stem Cell Rev and Rep (2024). https://doi.org/10.1007/s12015-024-10732-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s12015-024-10732-4